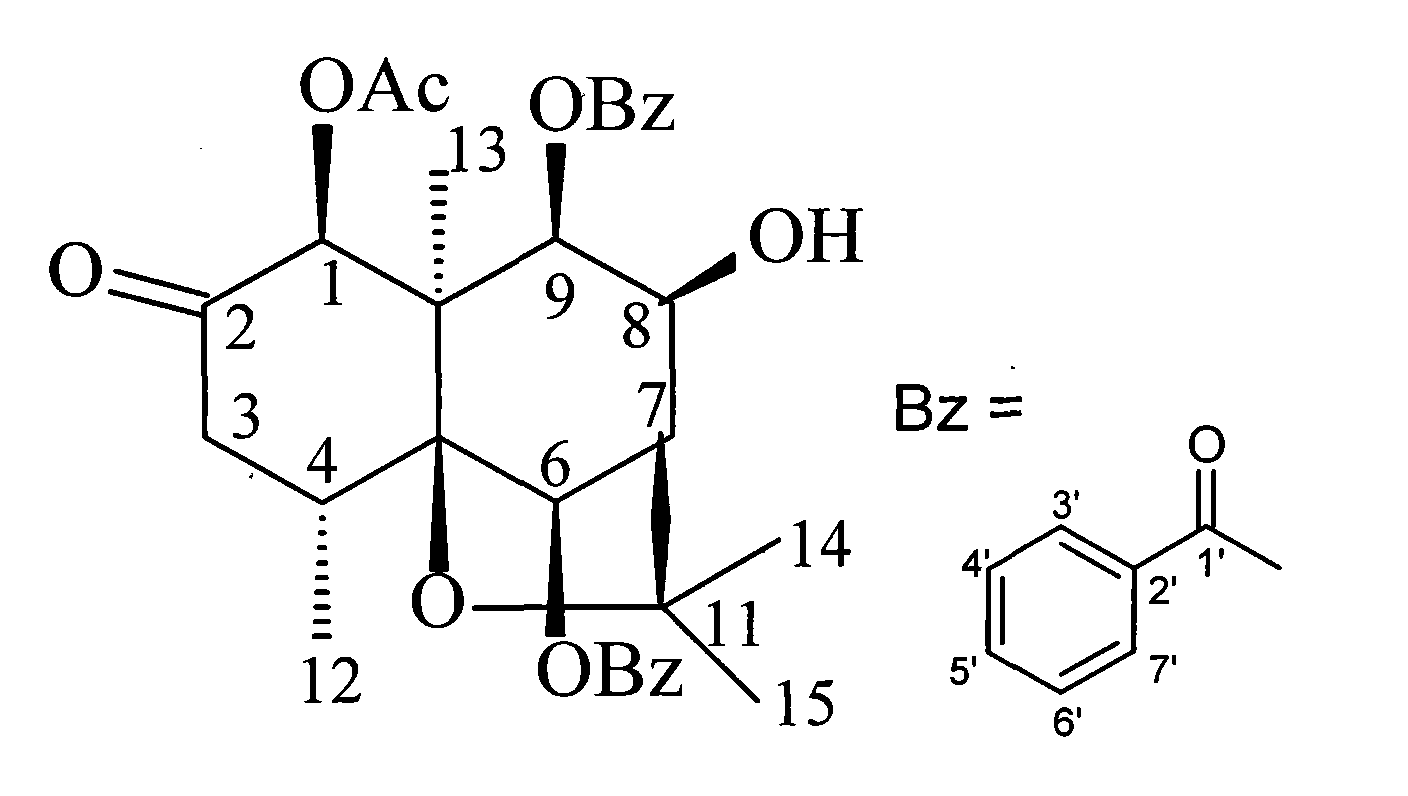
2-furoacridone-beta-dihydroagarofuran sesquiterpene compound in leafy parnassia, and preparation method and application thereof
A technology of dihydro agarwood furans and sesquiterpenes, which is applied in the field of medicine, can solve the research on the anti-tumor activity of 2-keto-β-dihydro agarwood furans compounds with complex and diverse structure types, complex and diverse substitution types, and 2-keto-β-dihydro agarwood furans. Issues that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Compound preparation
[0020] 1. Extraction
[0021] The dry weight of the whole herb of Xanthium chinensis is 1250g, and it is extracted with 95% ethanol at room temperature for 5 times, each time for 12 hours. After rotary evaporation to dryness, the ethanol phase extract was obtained, then suspended in water, followed by extraction with petroleum ether, ethyl acetate, and n-butanol to obtain 36.43 g of petroleum ether phase, 9.52 g of ethyl acetate phase, and 13.00 g of n-butanol phase. g and the aqueous phase 35.37 g.
[0022] Two, rough classification
[0023] After the ethyl acetate extract of cocklebur heptaacetate was suspended in water, it was extracted with petroleum ether, ether, ethyl acetate and n-butanol respectively to obtain C-E-1 (petroleum ether extraction layer), C-E-2 (ether extraction layer), C-E- 3 (ethyl acetate extraction layer, 5 g), C-E-4 (n-butanol extraction layer).
[0024] 3. Separation
[0025] Take 5 g of C-E-3 extract, dissolve with...
Embodiment 2
[0039] In vitro anti-tumor experiment
[0040] 1. Determination of tumor cell inhibition rate: MTT method
[0041] Tested cell lines: Two cell lines of human liver cancer cell HepG2 and human breast cancer cell line MDA-10 were selected.
[0042] Operation steps: Inoculate 2.5×10 per well in a 96-well plate 4 Each cell was incubated in a carbon dioxide cell incubator at 37°C for 6 hours, the culture solution in each well was sucked off, monomer compounds were added and cultured for 72 hours, stained with methylene blue staining, and the absorbance value was detected with a microplate reader. Investigate and compare the inhibitory rate of monomeric compounds on tumor cell proliferation.
[0043] 2. Test results
[0044] Table 2 Compounds inhibit the IC of different tumor cell proliferation 50 Value (μM)
[0045] Test sample
[0046] The test results showed that (1S, 4R, 5S, 6R, 7R, 8S, 9S, 10R)-1β-acetoxy-6β, 9β-dibenzoyloxy-2-keto-8β-hydroxyl-β- Dihydro agarwoo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com