N-(2)-L-alanyl-L-glutamine compound
A technology of glutamine and alanyl, which is applied in the field of N(2)-L-alanyl-L-glutamine compounds, can solve problems such as glutamine deficiency, and achieve good performance, solubility and Excellent stability, simple and controllable preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At 35°C, slowly add 4.0g of N(2)-L-alanyl-L-glutamine powder into 50ml of dioxane, stir to dissolve it, then stir the solution at this temperature for 14 hours, then cool down Crystals began to precipitate, and when the temperature was lowered to 5°C, a large amount of crystals precipitated, filtered, washed with propanol, dried, and 3.2 g of type III crystals were collected.
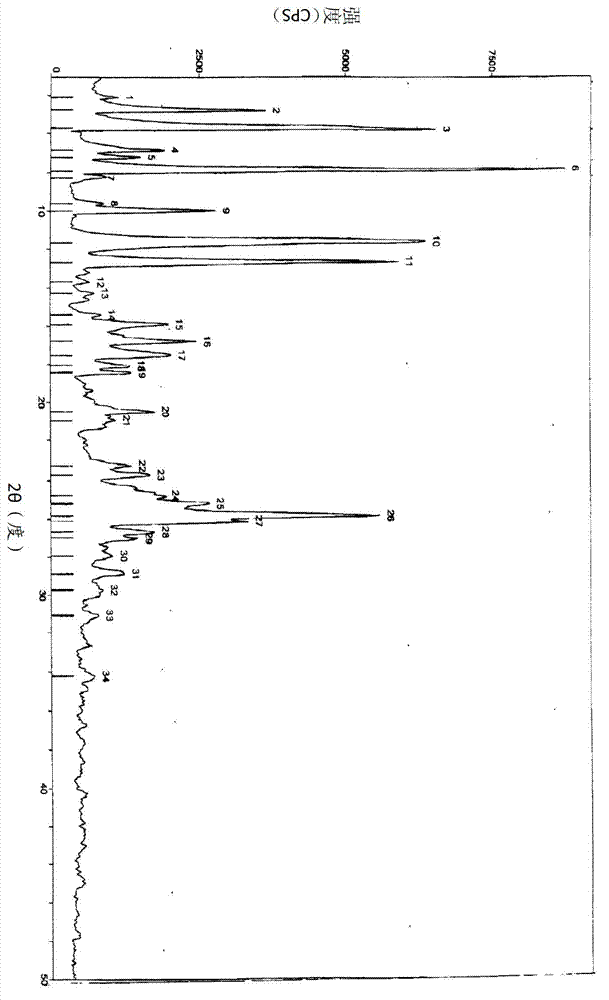
[0021] The powder X-ray diffraction pattern of the solid is shown in figure 1 shown. Powder X-ray diffraction was performed using the Scintag X2 Advanced Diffraction System (operated by ScintagDMS / NT 1.30a and Microsoft Windows NT 4.0 software). The system uses a Cu X-ray source (45kV and 40mA) to provide 1.5406 Cu Kα 1 ray, and a solid state Peltier cooled detector. The beam aperture was controlled using tube divergence and anti-scatter slits of 2 and 4 mm and detector anti-scatter and acceptance slits of 0.5 and 0.2 mm wide. Data were collected from 2 to 50° (2Θ) using a step scan with a ...
Embodiment 2
[0024] At 40°C, slowly add 5.0g of N(2)-L-alanyl-L-glutamine powder into 50ml of dimethylformamide, stir to dissolve it, and then stir the solution at this temperature for 12 After 1 hour, crystals began to precipitate when the temperature dropped to 8°C, a large amount of crystals precipitated, filtered, washed with propanol, dried, and 4.2 g of type III crystals were collected.
[0025] The powder X-ray diffraction pattern of the crystal is figure 1 resemblance.
Embodiment 3
[0027] At 50°C, slowly add 10.0g of N(2)-L-alanyl-L-glutamine powder into 100ml of dimethylformamide, stir to dissolve it, and then stir the solution at this temperature for 15 After 1 hour, crystals began to precipitate when the temperature dropped to 5° C., a large amount of crystals precipitated, filtered, washed with propanol, dried, and 8.3 g of type III crystals were collected.
[0028] The powder X-ray diffraction pattern of the crystal is figure 1 resemblance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com