Manufacture of hSCARB 2 transgenic mouse and application of the transgenic mouse as enterovirus infection animal model
An enterovirus and gene technology, applied in the field of preparation of enterovirus-infected animal models, can solve the problem of not being EV71
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
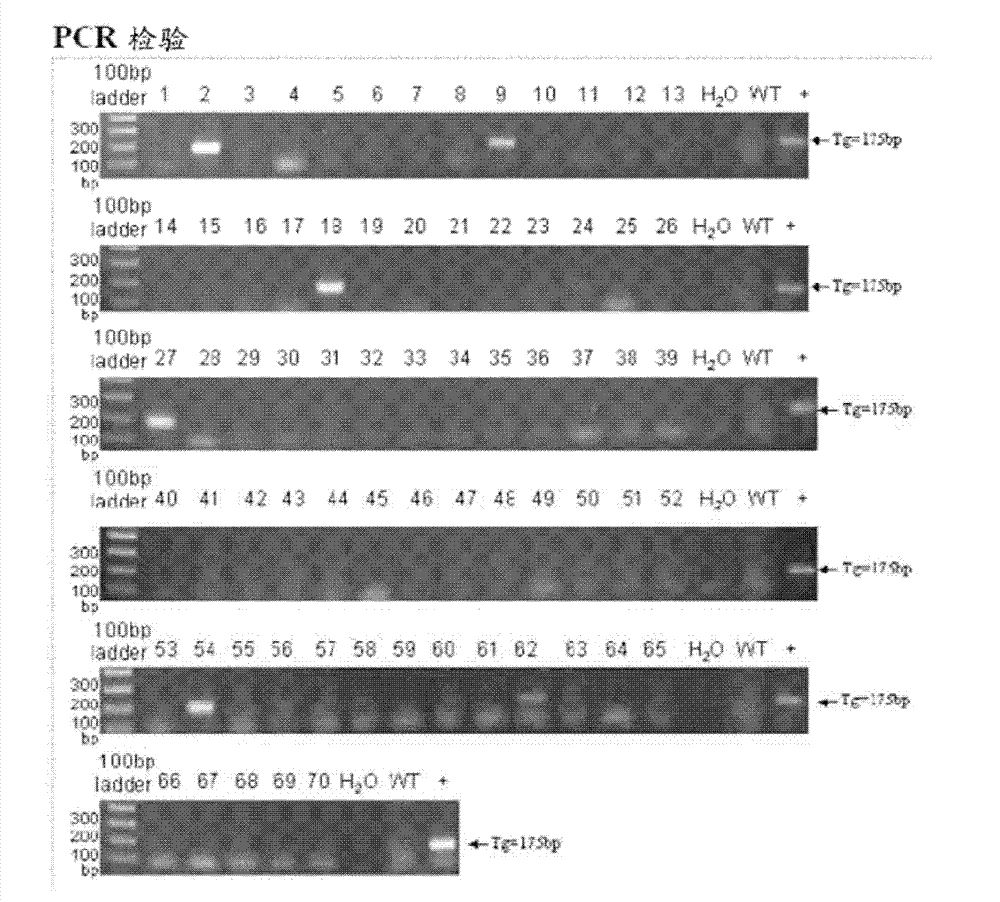
[0022] Example 1: Preparation of SCARB2 gene transgenic mice
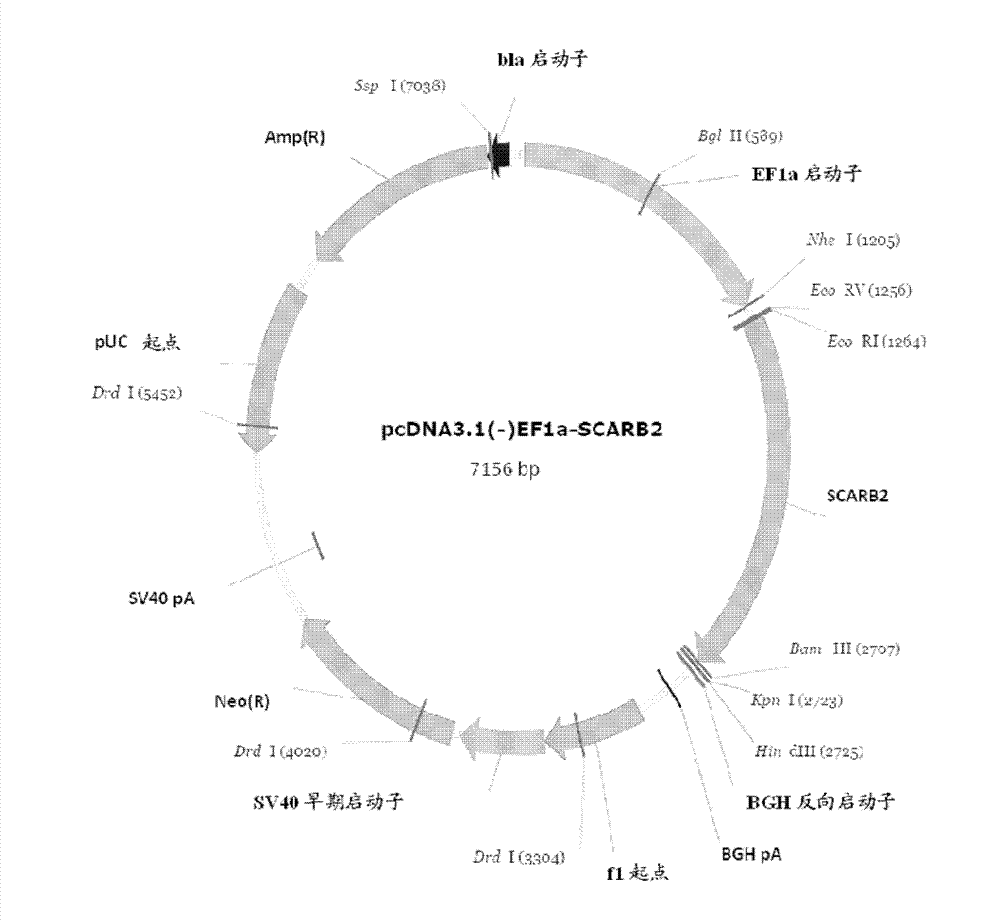
[0023] The synthesized codon-optimized human SCARB2 gene (SEQ ID NO.1) fragment was inserted into the pEF-1α plasmid (the plasmid was based on the pcDNA3.1 (-) vector (Invitrogen) using EcoRI and BamHI restriction enzymes. ) is a modified vector in which the original cytomegalovirus enhancer / promoter is replaced by the promoter of growth factor 1α (EF-1α), located in the promoter and bovine growth hormone (BGH) Multiple cloning sites between poly A tails were used to obtain the pEF-1α-hSCARB2 construct ( figure 1 ).
[0024]Generation of transgenic animals was performed according to the protocol described by Brinster et al. (Brinster et al., 1985). The C57BL / 6 mice used were purchased from National Applied Research Laboratories-Laboratory Animal Center (National Applied Research Laboratories-Laboratory Animal Center, Taiwan). Plastids were linearized with restriction enzymes prior to microinjection and recovered...
Embodiment 2
[0030] Example 2: Symptom analysis of viremia, hand-foot-mouth disease (HFMD) and nervous system lesions in SCARB2 gene transfected mice after enterovirus infection
[0031] 1-day or 4-day-old mice were subcutaneously injected into enterovirus EV71E59 or 5746-tw98 isolates (1x10 6 or 1x10 7 pfu, in 10 μL RPMI medium), to simulate the observation of the division of enterovirus in the main organs of the mouse after the newborn mouse was infected with the virus, and the external symptoms. The control group was given VP-SFM medium. For the detection of enterovirus EV71, the infected mice were sacrificed after a certain number of days, blood or organ tissue samples were collected, and specific real-time RT-PCR was performed for the VP1 gene.
[0032] Total RNA extracted from blood samples or tissue homogenates is converted into cDNA by reverse transcription. The resulting cDNA was then used with EV71 VP1 specific primer pair, forward primer: 5'-AGAGAGTCACTTGCTTGGCAGACA-3' (SEQ I...
Embodiment 3
[0037] Example 3: Using SCARB2 gene transfected mice to detect the prevention and treatment effect of anti-EV-71 antibody on enterovirus infection
[0038] In this example, the anti-EV-71 monoclonal antibody known to have enterovirus neutralizing activity was administered to SCARB2 gene-transformed mice previously infected with the natural EV71 5746-TW98 enteropathy isolate, and the monoclonal antibody was detected The effect of the antibody on protecting mice against enterovirus infection is to evaluate and prove the practicability of the SCARB2 gene transgenic mice of the present invention as a platform for screening anti-enterovirus drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com