Kit and method for detecting porous dental hydroxy apatite
A hydroxyapatite and kit technology, which can be used in the detection/measurement of irregular teeth, dental preparations, dentistry, etc., can solve the problems of difficult differential diagnosis and poor MIH effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
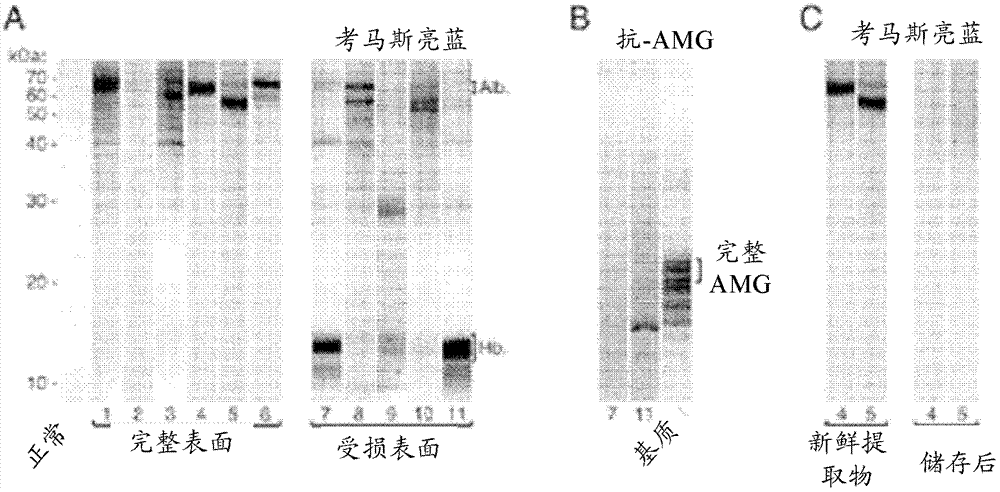
[0185] Example 2 - Preparation and testing of porous hydroxyapatite probes
[0186] Materials and Methods
[0187] SMCC (N-hydroxysuccinimidyl succinimidyl 4-[N-maleimidomethyl]cyclohexanecarboxylate; CAS#: 64987-85-5) is a non-cleavable isoform Bifunctional cross-linking agent, has been The spacer arm reactively separates the amine and sulfhydryl groups. Amino Black (CAS#: 1064-48-8) is a general purpose blue / black dye, used here as a chromogenic reporter containing primary amine groups. Hemoglobin from cow (CAS#: 9008-02-0) comprises a heterotetramer of 2 pairs of polypeptide chains (α and β; respectively: SEQ ID NO 20 and SEQ ID NO 21). The beta chain has a single solvent-exposed thiol-containing cysteine residue, while the alpha chain has no cysteine.
[0188] SMCC (75 mM in dimethyl sulfoxide) was added to 9 volumes of amino black (37.5 mM in phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM disodium hydrogen phosphate, pH 7.4)) and Incubate at 21°C...
Embodiment 3
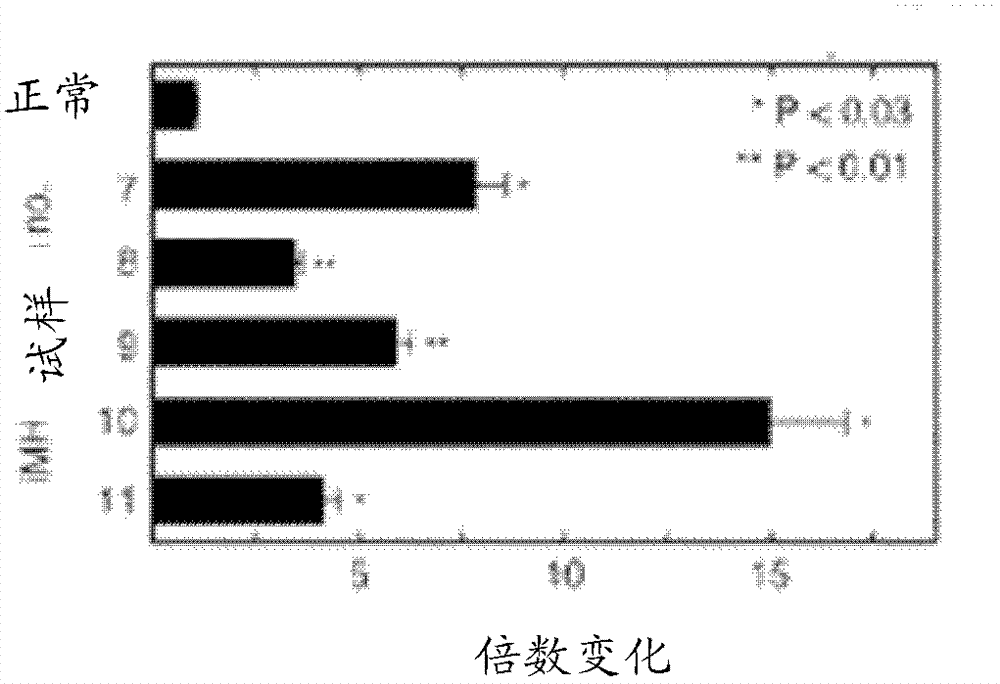
[0195] Example 3 - Probes that specifically bind to porous enamel ( Figure 30 )
[0196] method
[0197] To test whether the probe in Example 2 specifically binds to porous enamel, a complex carious lesion was covered with the probe and rinsed thoroughly.
[0198] Photograph of a human first molar with a large area of caries (porous enamel, white opaque areas) before and after application of the probe ( Figure 30 ). Use a brush to apply the probe to the entire crown area for 1 minute. After application of the probe, the tooth was rinsed under running water for 10 seconds, photographed, and then the tooth was rinsed again for 2 minutes, and photographed.
[0199] result
[0200] Normal enamel is not labeled.
[0201] Clearly carious areas were marked specifically and clearly, but the markings were patchy in some places. Unmarked carious areas show a bright surface that is resistant to scratching, while marked areas have a dark surface that is easily scratched. T...
Embodiment 4
[0204] Example 4 - The probe is able to specifically detect early demineralization of the enamel surface (incipient caries model) ( Figure 31 )
[0205] method
[0206] To test whether the probe in Example 2 could specifically detect early caries, artificial caries lesions were created (before probe application) on normal enamel surfaces using strong acid spots.
[0207] Human first molars, caries-free prior to acid treatment, were photographed before and after probe application ( Figure 31 ). Three enamel areas were then exposed to acid (0.5 μl 85% H 3 PO 4 ) for 1, 3 or 10 minutes to introduce artificial carious lesions, followed by a two-minute rinse in 100 ml TBS (25 mM Tris pH 7.2, 160 mM NaCl), followed by an additional 2-minute rinse under running water. The tooth was air dried and the probe was applied to the entire area with a brush for 3 minutes. After application, unbound probes were first removed by wiping with absorbent paper and then rinsed under runnin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com