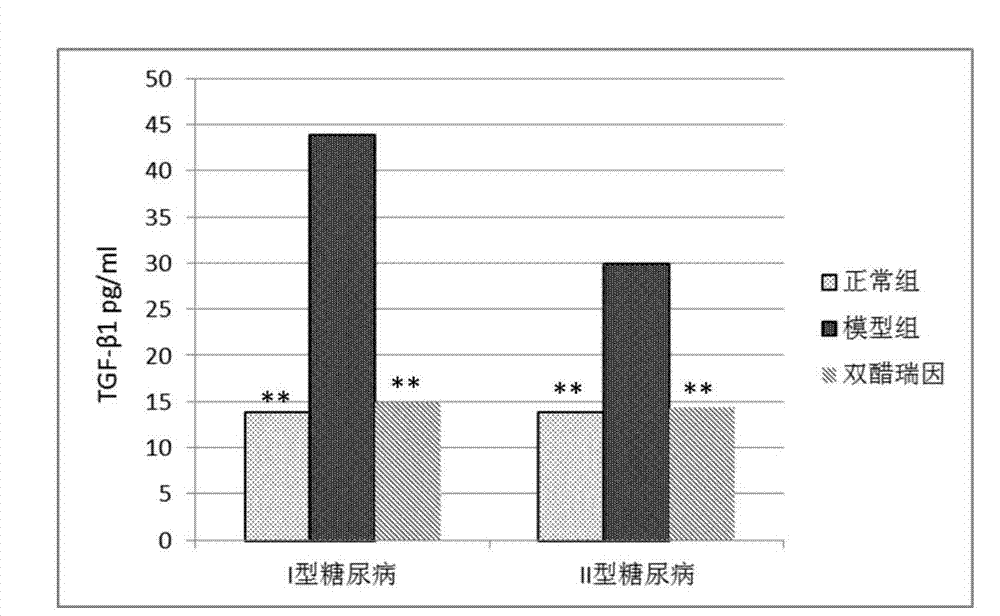
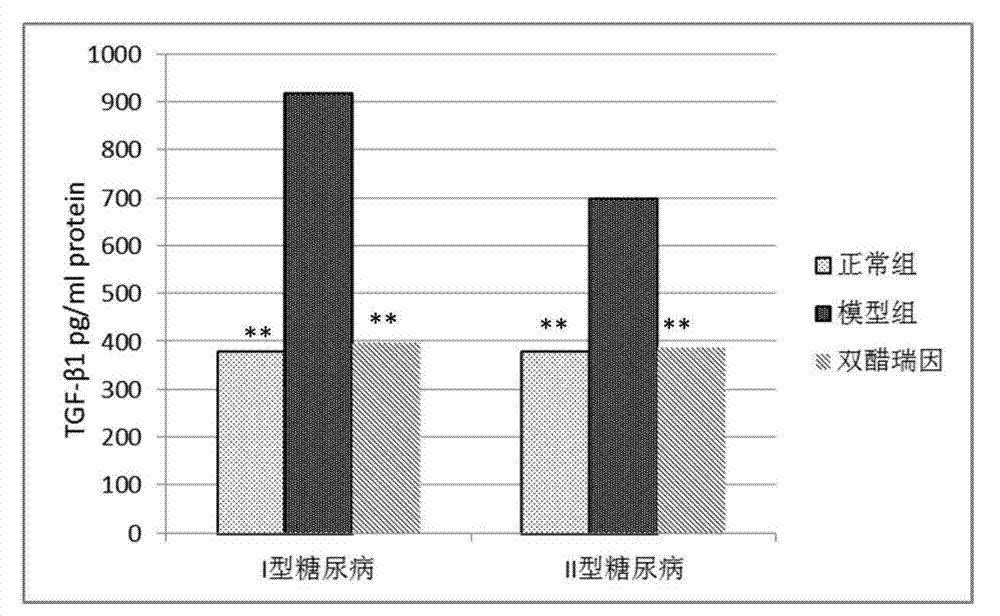
Application of diacerein to prepare medicine for treating diabetic nephropathy
A technology of diabetic nephropathy and diacerein, which is applied in drug combinations, urinary system diseases, metabolic diseases, etc., can solve the problems of unreported use of diacerein, and achieve the treatment of non-specific vascular injury and interstitial injury, Improve the effect of renal pathological changes and insulin resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0058] Experimental example 1. Therapeutic effect of diacerein on alloxan-induced type I diabetes in mice.
[0059] Experimental method: Alloxan 80 mg / kg was injected into the tail vein of Kunming mice weighing 20±1 g, once a week, for a total of 2 injections. One week after the last injection, blood was collected from the tail to measure fasting blood glucose, and mice with fasting blood glucose greater than 13 mmol / L were regarded as type I diabetic mice.
[0060]The selected mice were randomly grouped according to the level of blood sugar: model control group: intravenous injection or intragastric administration of the same volume of solvent; treatment group: intragastric administration, diacerein 100mg / kg, 10 mice in each group, half male and half male , administer once a day, measure the food intake and water intake of the mice every day, draw the change curve of the weekly food intake and water intake of the mice, and continue for 10 weeks. At the end of the 6th week an...
experiment example 2
[0063] Experimental example 2, the therapeutic effect of diacerein on type 2 diabetes mellitus induced by streptozotocin
[0064] Experimental method: Streptozotocin 100 mg / kg was injected into the tail vein of C57BL / 6 mice weighing 20±1 g, and nicotinamide 100 mg / kg was injected intraperitoneally, once a week, for a total of 3 injections. One week after the last injection, blood was collected from the tail to measure fasting blood glucose, and mice with fasting blood glucose of 9-12 mmol / L were regarded as type II diabetic mice.
[0065] The selected mice were randomly grouped according to the level of blood sugar: model control group: intravenous injection or intragastric administration of the same volume of solvent; treatment group: intragastric administration, diacerein 100mg / kg, 10 mice in each group, half male and half male , administered once a day for 8 consecutive weeks. At the end of the 5th week and the 8th week of treatment, the fasting blood glucose was measured ...
experiment example 3
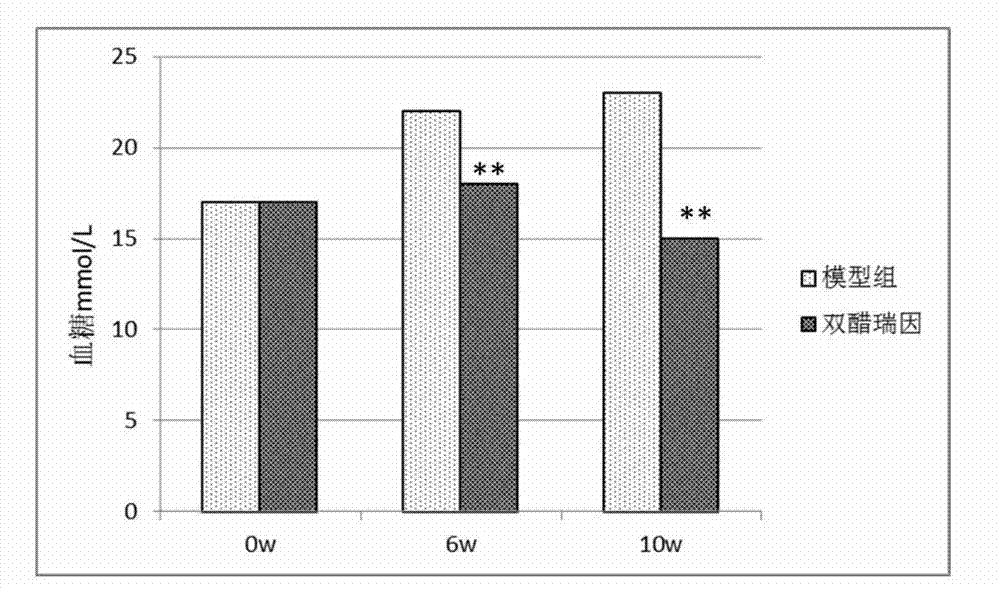
[0068] Experimental Example 3, Preventive Effect of Diacerein Alloxan-Induced Type I Diabetes in Mice
[0069] The establishment of the diabetes model was the same as in Experiment 1, and the drug was administered from 7 days before the establishment of the model. The dose was the same as in Experiment 1, and the drug was administered once a day.
[0070] Results: Diacerein can prevent alloxan-induced increase in blood glucose in mice with type I diabetes (see Figure 9 ).
[0071] The above results indicated that diacerein had a preventive effect on alloxan-induced type I diabetes in mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com