Apoptosis imaging agents based on lantibiotic peptides
A technology of lantibiotics and imaging agents, applied in the directions of in vivo radioactive preparations, preparation of X-ray contrast agents, preparations for in vivo experiments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
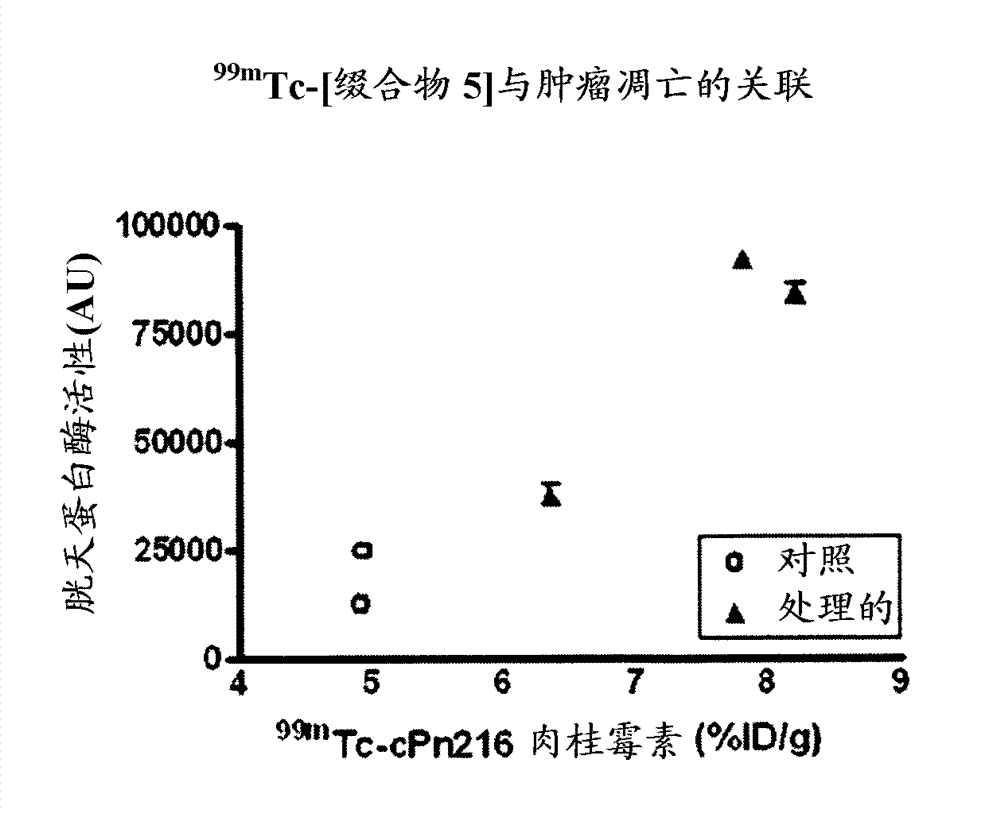
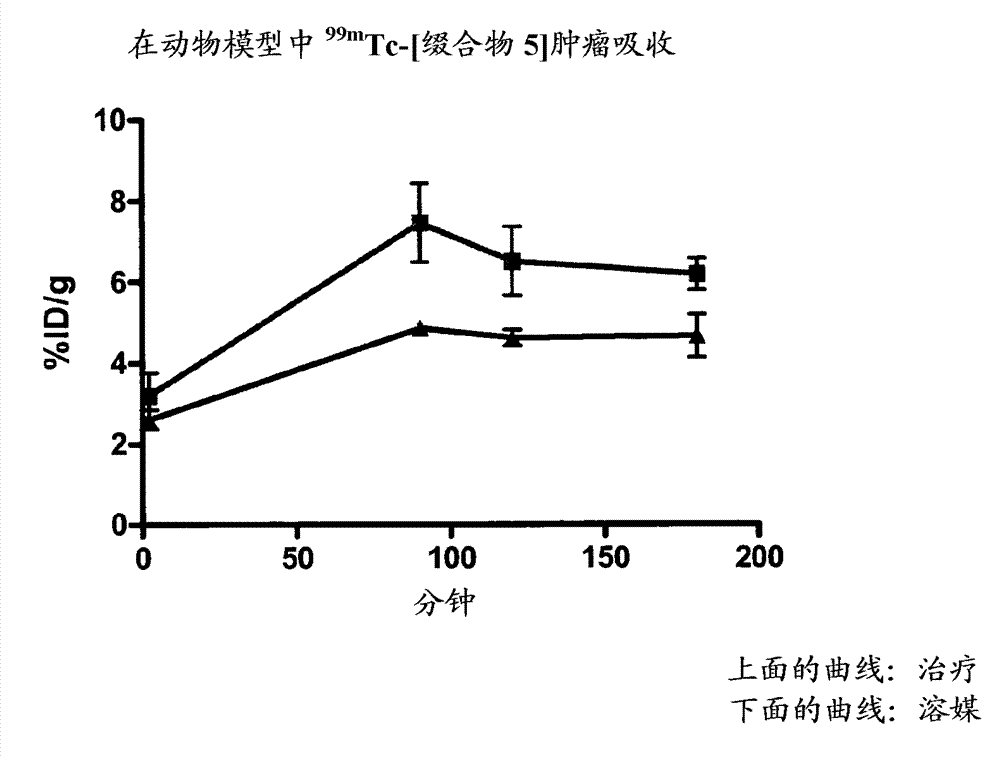
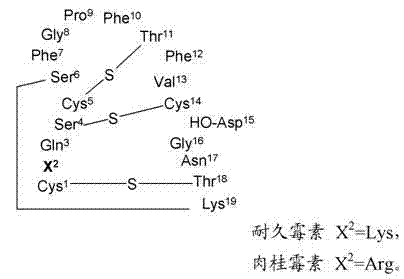
[0154] Example 15 provides the determination of the conjugation site of the chelator, and Example 16 demonstrates that the conjugation site of the chelator has a significant effect on the binding affinity to phosphatidylethanolamine with an 18-fold difference (K d 5 nM vs. 90 nM). This provides a preference for linkage at the N-terminus (Cys of Formula II) than at Xaa of Formula II. a ) evidence of attachment of a radiometal complex. The EL4 lymphoma mouse xenograft tumor model of Example 17 was used as a model to mimic the apoptotic response following chemotherapy. Treatment-treated mice (etoposide / cyclophosphamide) showed a 4-fold increase in tumor apoptosis compared to vehicle control-treated animals. The biodistribution results of Example 17 showed higher uptake of each agent in chemotherapy-treated tumors, while correlation analysis indicated a trend towards higher uptake of the binders in tumors with higher levels of apoptosis. compared to 99m Tc-[conjugate 3A], 99...
Embodiment 1
[0194] Example 1: Synthesis of 1,1,1-tris(2-aminoethyl)methane.
[0195] Step 1(a): Dimethyl 3(methoxycarbonylmethylene)glutarate.
[0196] Carbomethoxymethylenetriphenylphosphine (167g, 0.5mol) in toluene (600ml) was treated with dimethyl 3-oxoglutarate (87g, 0.5mol) at 120°C under nitrogen atmosphere On an oil bath, the reaction was heated to 100°C for 36 hours. The reaction was then concentrated in vacuo and the oily residue was triturated with 40 / 60 petroleum ether / diethyl ether 1:1 (600ml). Triphenylphosphine oxide precipitated out and the supernatant liquid was decanted / filtered. The vacuum evaporated residue was Kugel distilled under high vacuum Bpt (oven temperature 180-200°C, 0.2 Torr) to give dimethyl 3-(methoxycarbonylmethylene)glutarate (89.08 g , 53%).
[0197] NMR 1 H(CDCl 3 ): δ 3.31 (2H, s, CH 2 ), 3.7 (9H, s, 3×OCH 3 ), 3.87 (2H, s, CH 2 ), 5.79 (1H, s, =CH) ppm.
[0198] NMR 13 C(CDCl 3 ), δ 36.56, CH 3 , 48.7, 2×CH 3 , 52.09 and 52.5 (2×CH 2 )...
Embodiment 2
[0223] Example 2: Preparation of 3-chloro-3-methyl-2-nitrosobutane.
[0224] A mixture of 2-methylbut-2-ene (147ml, 1.4mol) and isopentylonitrile (156ml, 1.16mol) was cooled to -30°C in a bath of cardice and methanol and stirred vigorously using a headspace air stirrer , was treated dropwise with concentrated hydrochloric acid (140ml, 1.68mol) at such a rate that the temperature was kept below -20°C. This takes about 1 hour due to the significant exotherm and care must be taken to prevent overheating. Ethanol (100 ml) was added to reduce the viscosity of the slurry formed at the end of the addition, and the reaction was stirred for an additional 2 hours at -20 to -10°C to complete the reaction. The precipitate was collected by filtration under vacuum, washed with 4 x 30 ml of cold (-20 °C) ethanol and 100 ml of ice-cold water, and dried in vacuo to give 3-chloro-3-methyl-2-nitroso as a white solid butane. The ethanol filtrate and washings were combined, diluted with water (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com