Carbidopa/levodopa/folic acid compound medicine composition and use thereof
A technology of levodopa and a composition is applied in the field of pharmacy to achieve the effects of reducing stroke, increasing curative effect, preventing and stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
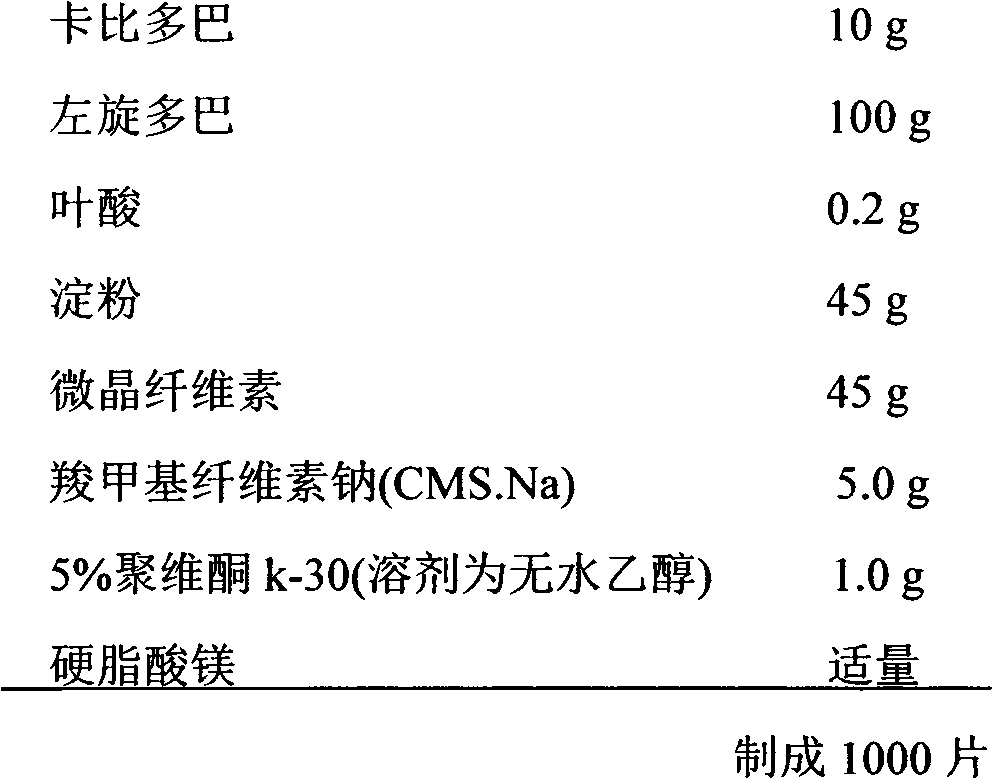
[0034] Embodiment 1. Preparation of tablet
[0035] prescription:
[0036]
[0037] Preparation Process:
[0038] (1) Take the carbidopa, levodopa and folic acid of the prescribed amount and pass through a 100 mesh sieve and mix them uniformly by the method of equal increments for subsequent use;
[0039] (2) Other auxiliary materials are passed through a 100-mesh sieve and dried at 75°C for 2 hours;
[0040] (3) Mix the starch, microcrystalline cellulose, and CMS.Na according to the prescription amount, and then mix them evenly with the mixed raw material drug in equal increments;
[0041] (4) Add an appropriate amount of binder to prepare soft materials, granulate with a 24-mesh sieve, granulate with a 20-mesh sieve, and dry at 40-75°C.
[0042] (5) Add an appropriate amount of magnesium stearate to the dry granules and mix evenly. After the content is determined, compress into tablets and pack.
[0043] 3 times a day, 1 tablet each time.
Embodiment 2
[0044] Embodiment 2. The preparation of capsule
[0045] prescription:
[0046]
[0047] Preparation Process:
[0048] According to the prescription ratio, take lactose, microcrystalline cellulose, starch, and sodium starch glycolate and dry them at about 100°C for about 2 hours to pass through a 100-mesh sieve; after passing the raw material through a 100-mesh sieve, mix with the above-mentioned excipients in equal amounts Evenly, capsule filling.
[0049] 3 times a day, 1 capsule each time.
Embodiment 3
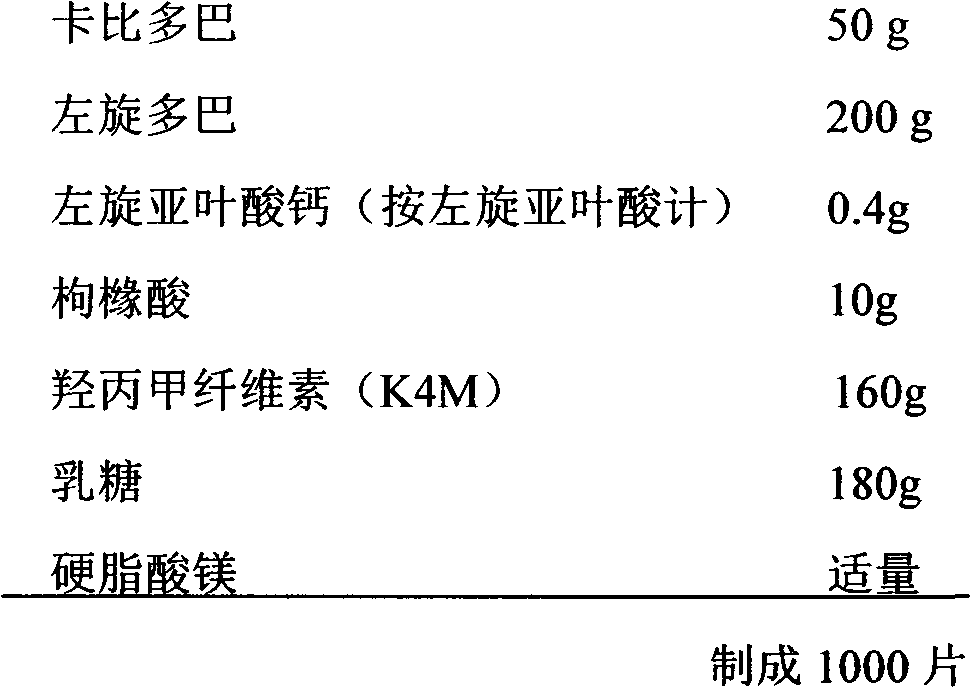
[0050] Example 3. Preparation of Sustained Release Tablets
[0051] prescription:
[0052]
[0053] Preparation Process:
[0054] According to the proportion of the prescription, the raw material is mixed with hypromellose, citric acid is dissolved in ethanol as a wetting agent to make a soft material, granulated, dried, sized, mixed with magnesium stearate, pressed Slice and serve.
[0055] 2 to 3 times a day, 1 tablet each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com