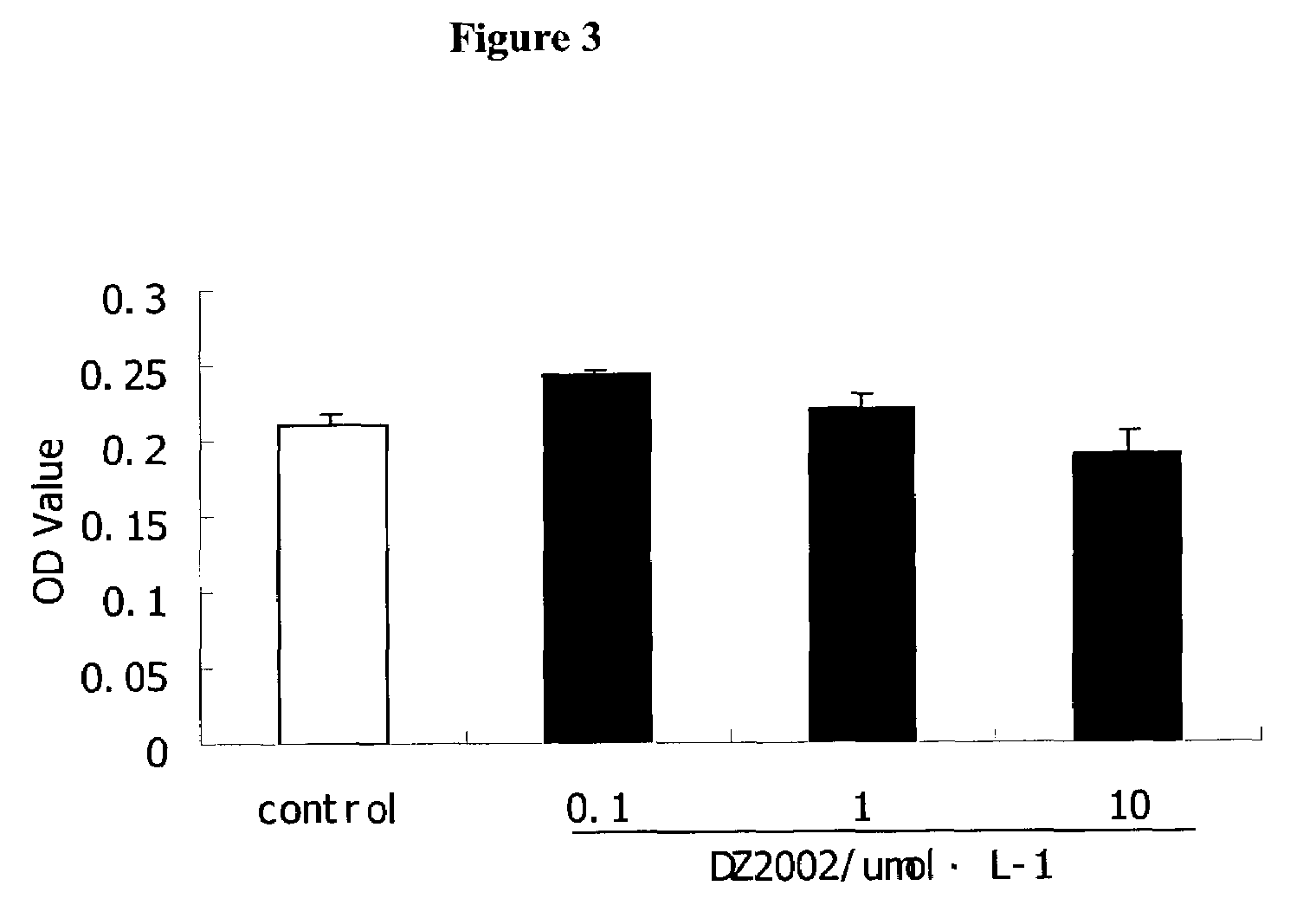
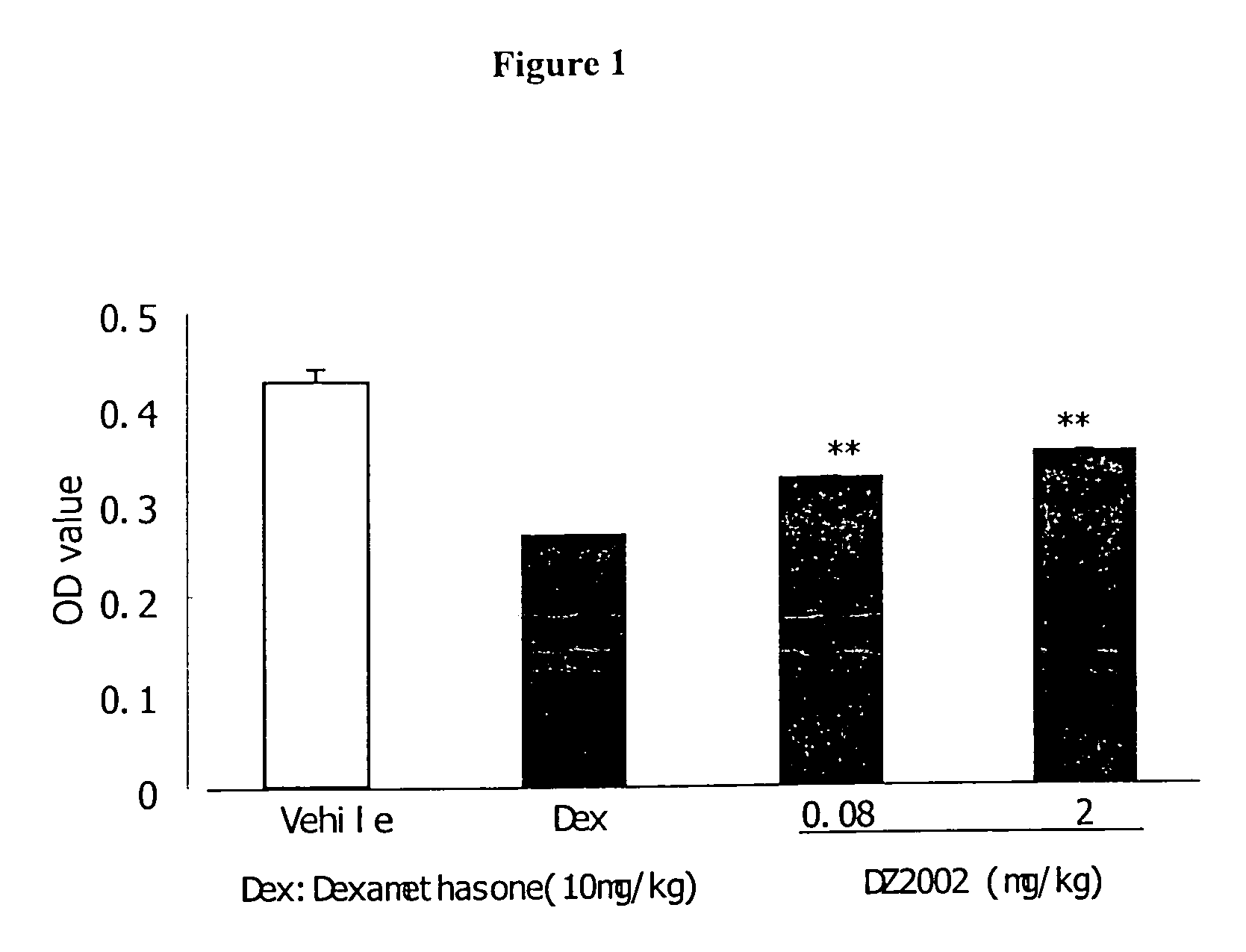
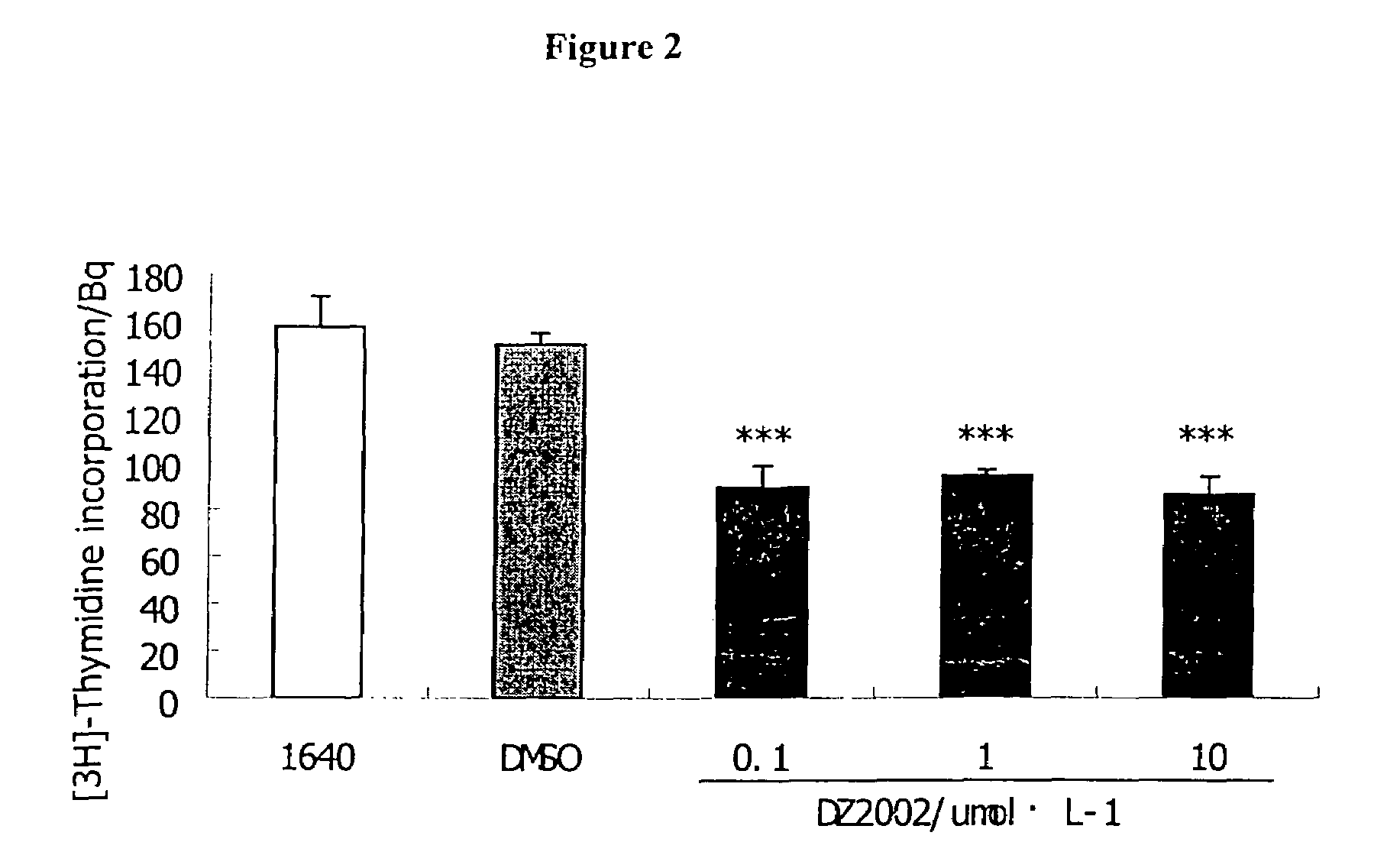
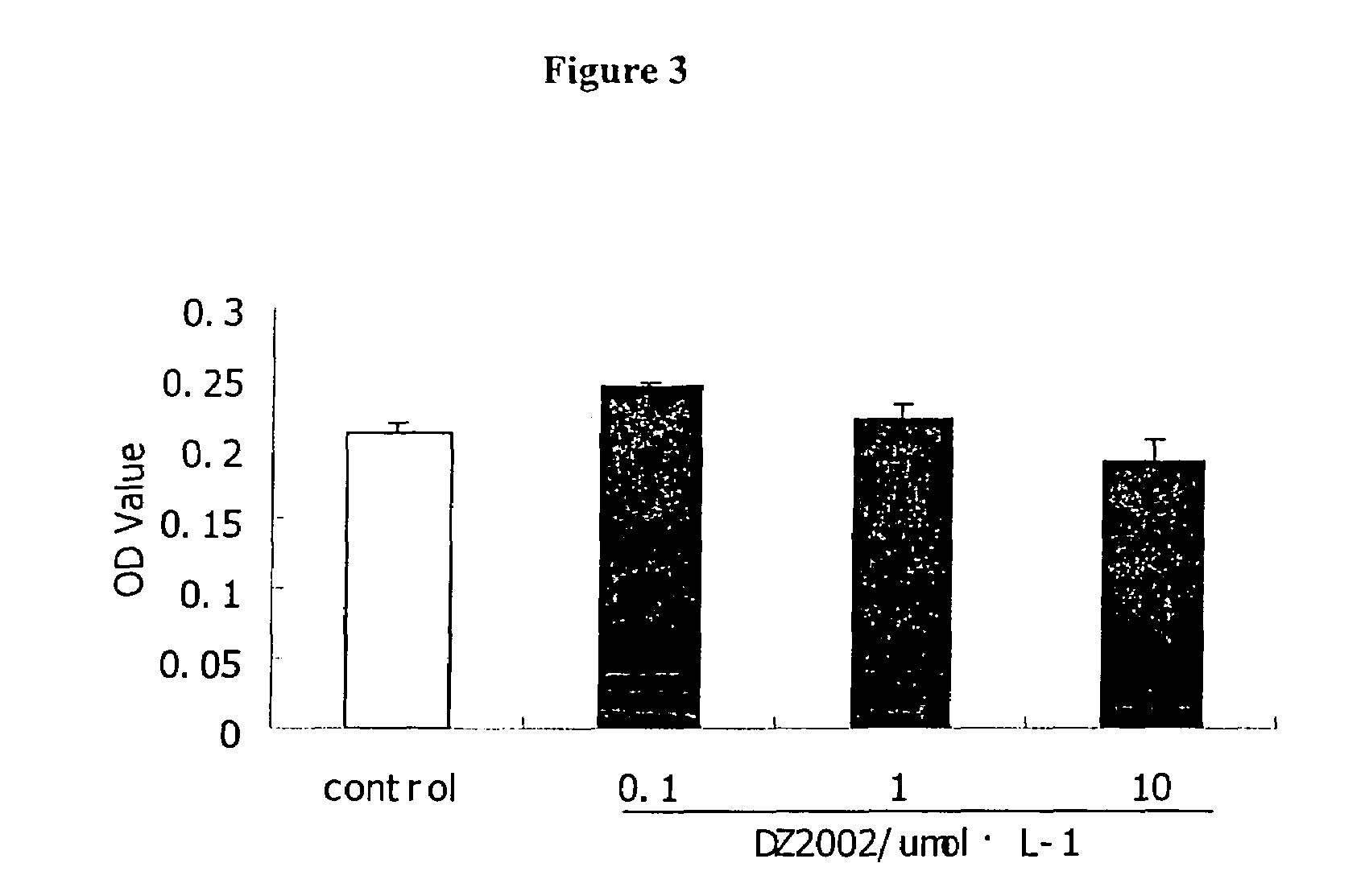
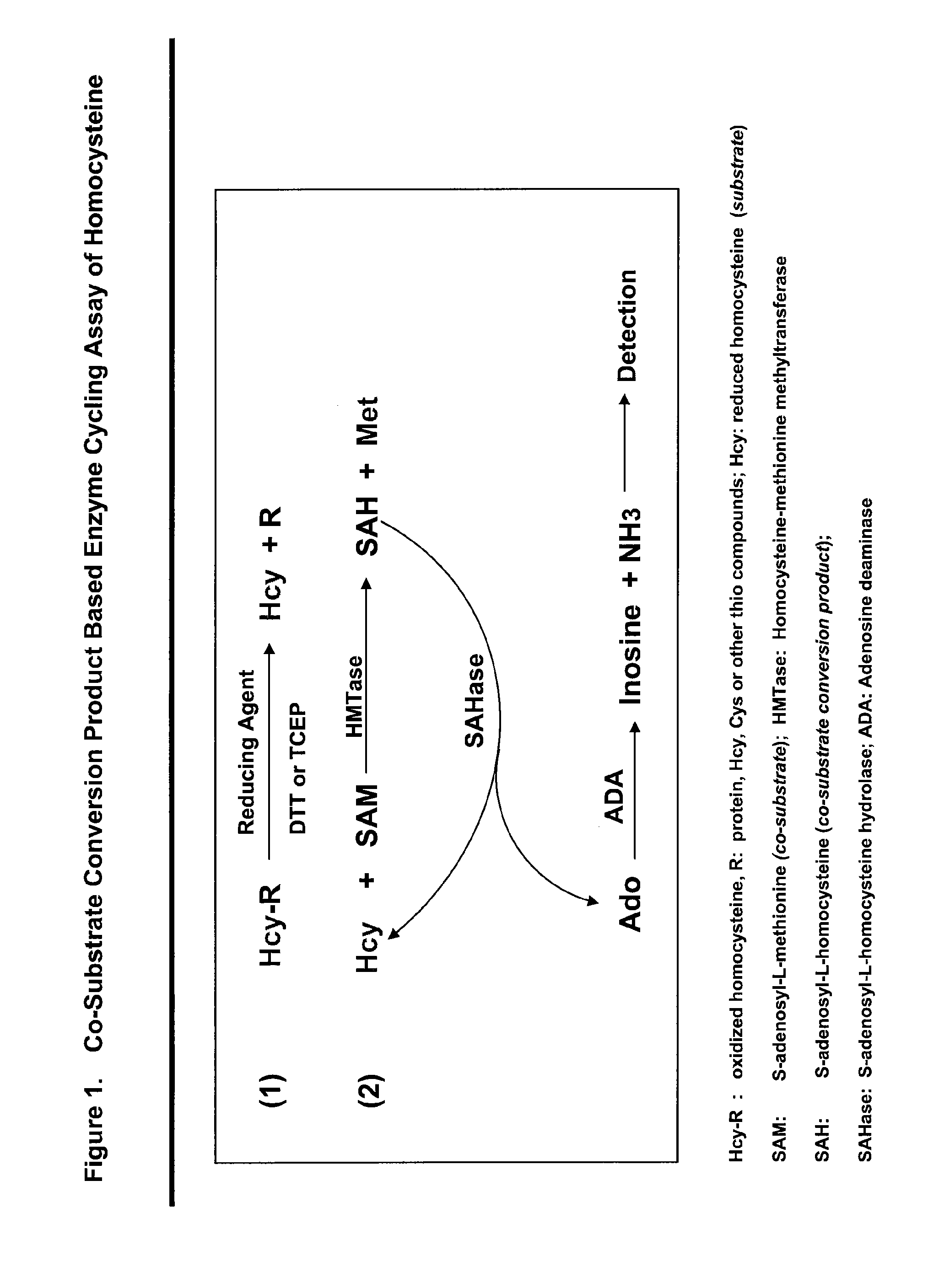
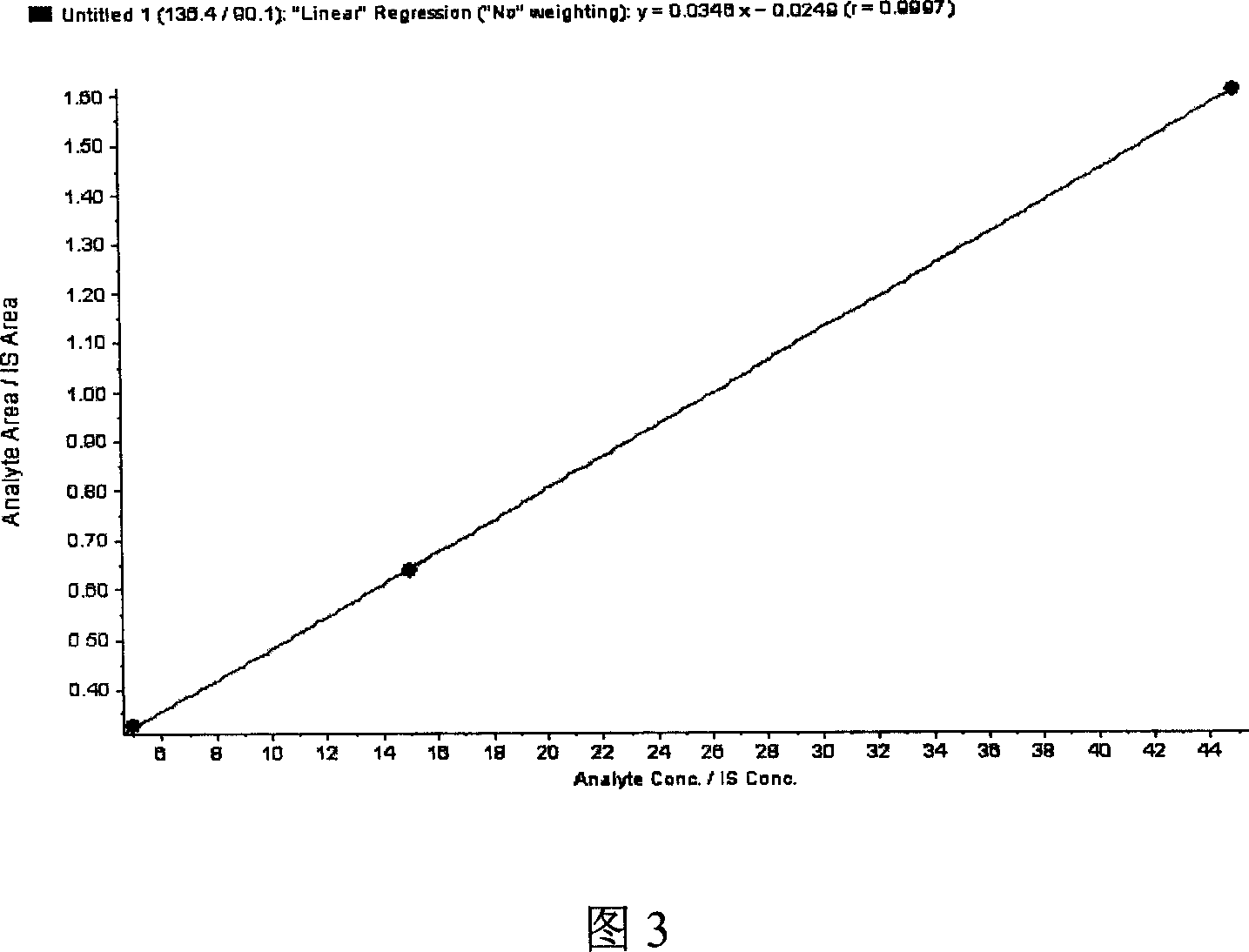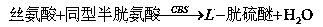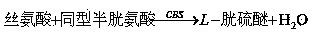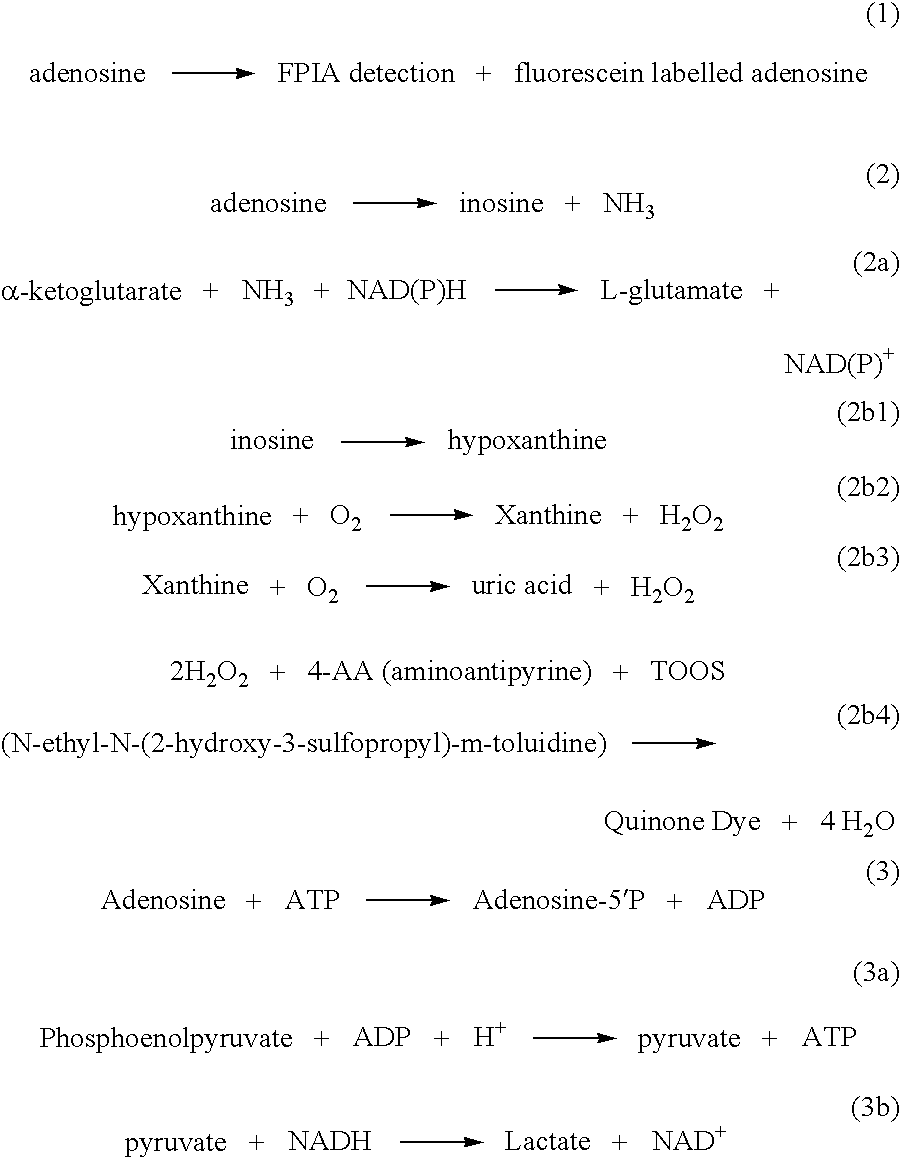Patents
Literature
113 results about "Hcy homocysteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
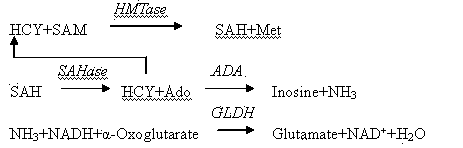
Homocysteine (HCY) Hyperhomocysteinemia is a medical condition characterized by elevated levels of the intermediate amino acid, homocysteine (HCY), in the blood. 1 HCY plays a key role in the remethylation/transmethylation and transsulfuration pathways that require folate, cobalamin, and pyridoxine as cofactors.
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Ketone bodies and ketone body esters as blood lipid lowering agents
ActiveUS9211275B2Reduce serum cholesterol and/or triglyceride levelLowering of total serum cholesterol levelHydroxy compound active ingredientsMetabolism disorderChemistryNutritional composition
The subject disclosure provides compositions for reducing serum cholesterol and / or triglyceride levels in subjects. These compositions can comprise racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol or R-1,3 butandiol alone and can be, optionally, administered in conjunction with a low fat diet to a subject. Alternatively, compositions comprising racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol, R-1,3 butandiol or combinations thereof can be formulated as nutritional supplements (also referred to as nutritional compositions) or incorporated into therapeutic compositions containing a) anti-hypertensive agents; b) anti-inflammatory agents; c) glucose lowering agents; or d) anti-lipemic agents) which are administered to a subject, optionally in combination with a low fat diet, in order to cause a reduction or lowering of: serum cholesterol levels; triglyceride levels; serum glucose levels, serum homocysteine levels, inflammatory proteins (e.g., C reactive protein) and / or hypertension in treated subjects. Alternatively, compositions disclosed herein can be administered alone, or in combination with other therapeutic agents to prevent or reverse vascular disease.
Owner:OXFORD UNIV INNOVATION LTD +1
Reversible inhibitors of S-adenosyl-L-homocysteine hydrolase and uses thereof
The present invention provides compositions and methods for reversibly inhibiting S-adenosyl-L-homocysteine (SAH) hydrolase. The compounds of the present invention can be used in combination with an anti-hemorrhagic viral infection agent, an immunosuppressant, a homocysteine lowering agent, or an anti-neoplasm agent. The compositions and methods of the present invention can be used for the prevention and treatment of hemorrhagic virus infection, autoimmune diseases, autograft rejection, neoplasm, hyperhomocysteineuria, cardiovascular disease, stroke, Alzheimer's disease, or diabetes.
Owner:NINGBO ZIYUAN PHARMA INC
Usages of MTHFR gene polymorphisms in predicting homocysteine level, disease risk, and treatment effects and related methods and kit
InactiveUS20070134709A1Improve the level ofBiocideBioreactor/fermenter combinationsDisease riskTreatment effect
This invention features our discovery on usages of Methylenetetrahydrofolate Reductase (MTHFR) gene polymorphisms in predicting homocysteine (Hcy) level and / or incidence and prognosis of diseases associated with increased Hcy level in a subject, as well as predicting treatment effects of medicines in the category of Angiotension Converting Enzyme Inhibihor (ACEI) with and without combination with B Vitamins. This invention also features our discovery on laboratory and analytical methods that are essential to the above described usages of MTHFR gene polymorphisms. In addition, this invention features a kit that has translated the above discoveries into a practical and reliable tool that can be applied to accomplish the above described usages of MTHFR gene polymorphisms. This invention represents an important step in realizing personalized medicine, with the goal to tailor diagnosis, prevention and treatment strategy to meet individual needs.
Owner:XU XIPING +14
Reversible inhibitors of SAH hydrolase and uses thereof
1. The present invention provides compositions and methods for reversibly inhibiting S-adenosyl-L-homocysteine (SAH) hydrolase. The compounds of the present invention can be used as an anti-hemorrhagic viral infection agent, an immunosuppressant, a homocysteine lowering agent, or an anti-neoplasm agent. The compositions and methods of the present invention can be used for the prevention and treatment of hemorrhagic virus infection, autoimmune diseases, autograft rejection, neoplasm, hyperhomocysteineuria, cardiovascular disease, stroke, Alzheimer's disease, multiple sclerosis or diabetes. The compound of the present invention and / or used in the present invention has the formula (I):wherein Z is selected from the group consisting of carbon and nitrogen,R1 and R2 are the same or different, and are selected from the group consisting of hydrogen, hydroxy, alkyl, cycloalkyl, alkenyl, alkoxy, amino, aryl, heteroaryl, and halogen;R3 and R4 are the same or different and are selected from the group consisting of hydrogen, alkyl, acetyl, alkenyl, aryl, and heteroaryl;X is selected from the group consisting of oxygen, nitrogen, and sulfur; andY is selected from the group consisting of hydrogen, a C1-10 alkyl group, alkenyl, vinyl, aryl, and heteroaryl, provided that the compound is not (4-adenine-9-yl)-2-hydroxybutanoic acid.
Owner:NINGBO ZIYUAN PHARMA INC
Intra-intestinal nutrient emulsion for tumor patients
InactiveCN103609933ANutritional balanceRecovery functionMetabolism disorderEmulsion deliveryNutritionTryptophan
The invention belongs to the medicine field, and specifically relates to an intra-intestinal nutrient emulsion for tumor patients. The emulsion is characterized by comprises the following effective components: protein, fat, carbohydrate, total dietary fiber, green tea theine, taurine, L-carnitine, composite vitamins and mineral substances, and water. The protein is provided in an amino acid powder form, the formula of the amino acid powder is adjusted on the basis of 20 kinds of protein amino acids, wherein methionine is replaced by homocysteine, glutamine and glutamic acid are not added, the tryptophan content is low, and the content of branched chain amino acid reaches 35% or more; fat is provided in a saturated aliphatic acid or unsaturated fatty acid form, wherein in the unsaturated aliphatic acid the value of omega-6 / omega-3 is equal to 2-6 / 1; and the carbohydrate is provided in a form of glycerin, glucose, fructo-oligosaccharide or lentinan. Besides one prominent characteristic of the emulsion is that the emulsion is a high-fat and low-sugar type, and the ratio of sugar to fat is 1:1. The emulsion formula is adjusted according to the nutrient and metabolism requirements of tumor patients so as to develop an individualized intra-intestinal nutrient preparation for tumor patients, the preparation can provide balanced nutrients for tumor patients, effectively cures the malnutrition symptom, improves the life quality of patients, and can also be used to assist the tumor treatment.
Owner:湖北一半天制药有限公司
Rapid multiple panel of biomarkers in laboratory blood tests for TIA/stroke
InactiveUS20060024749A1Volume andPeptide/protein ingredientsMicrobiological testing/measurementNR1 NMDA receptorNMDA receptor
Owner:CIS BIOTECH
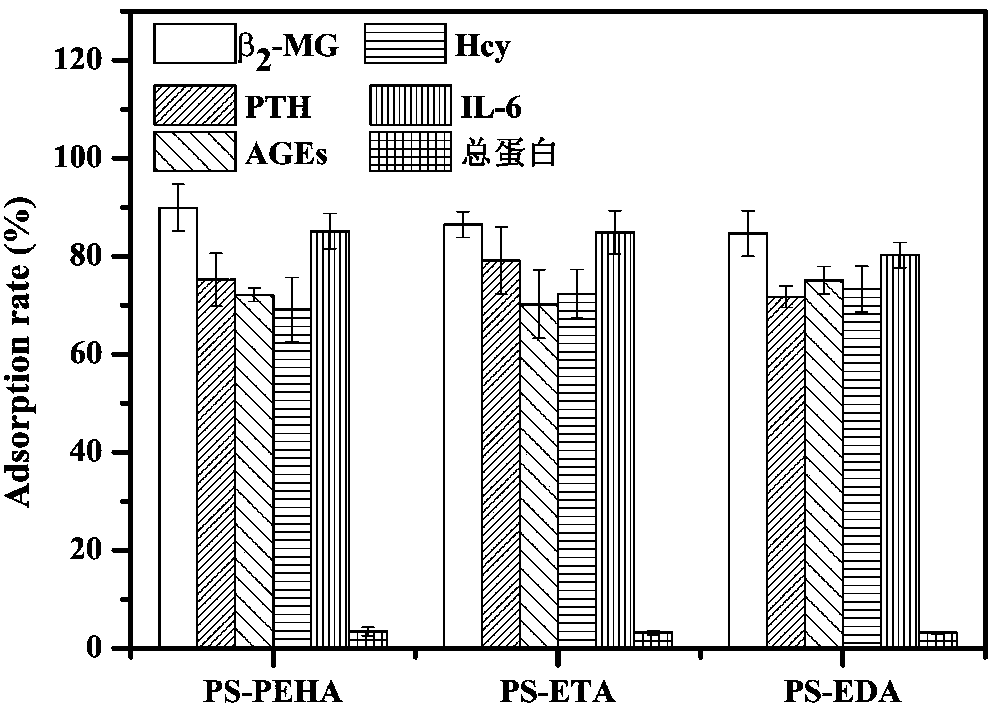
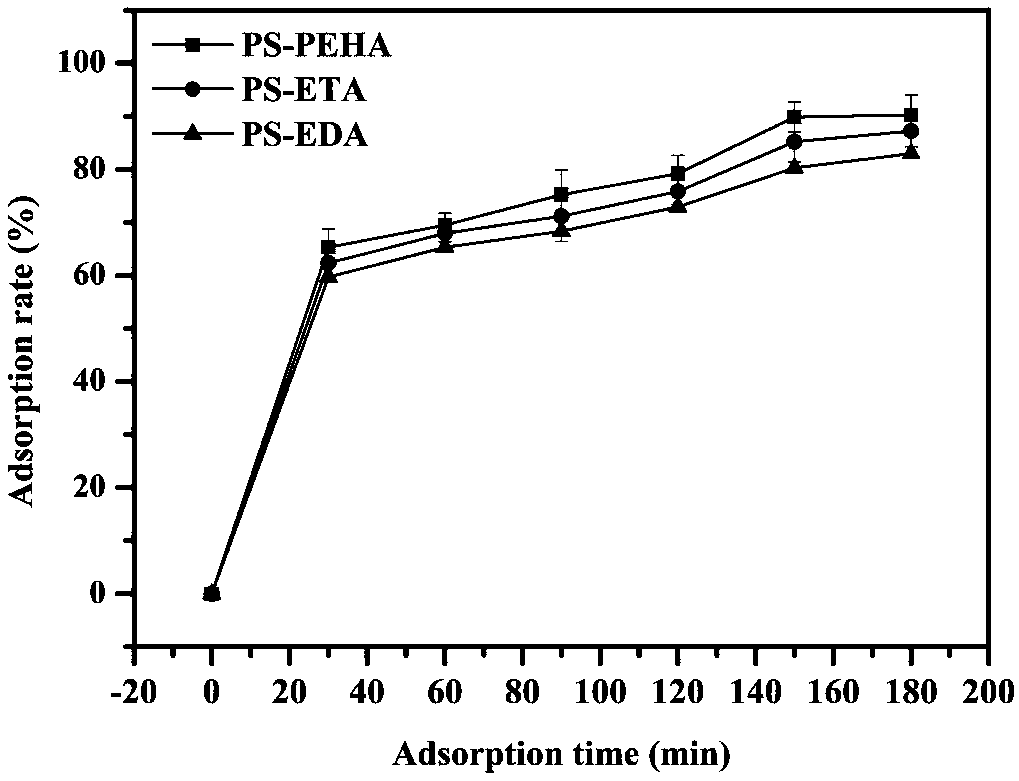
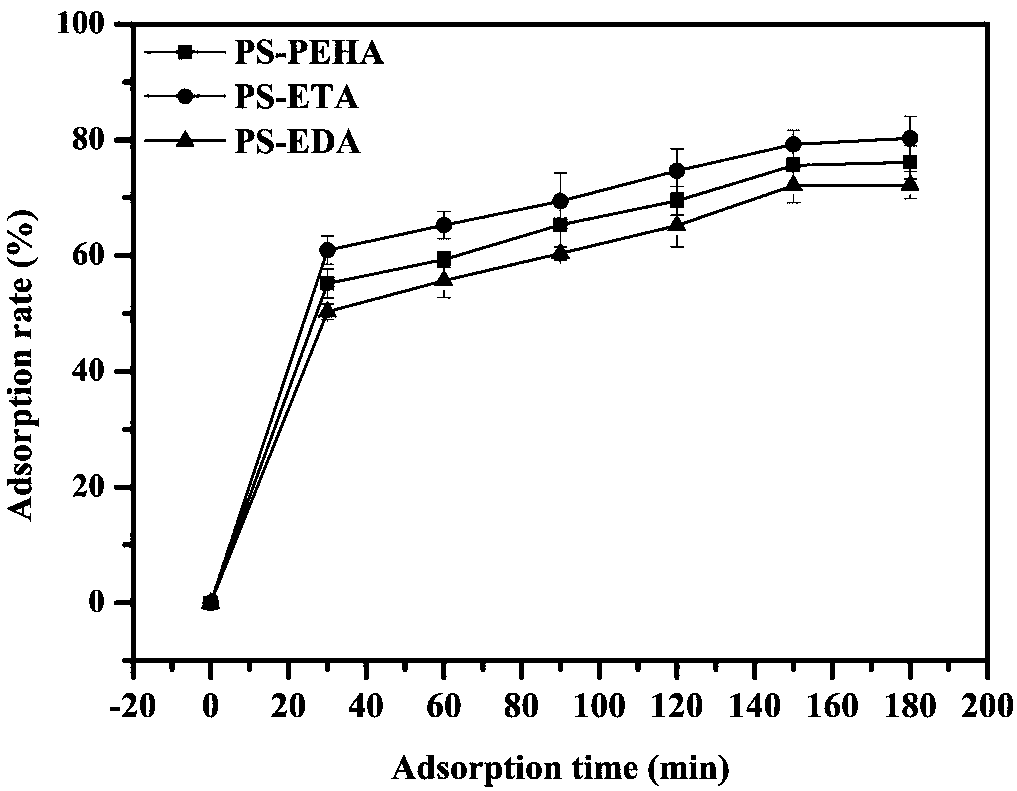
Adsorbent used for clearing middle and macro molecule toxin in body of uremia patients and preparation method thereof
ActiveCN108371945AEfficient removalHigh adsorption selectivityOther chemical processesOther blood circulation devicesSorbentPolystyrene
The invention relates to an adsorbent used for clearing middle and macro molecule toxin in body of uremia patients and a preparation method thereof. According to the molecule size of middle and macromolecules such as beta2-MG, PTH, AGEs, Hcy and IL-6 and performance characteristics thereof, a functional adsorbent which can efficiently clear main middle and macro molecule toxins such as beta2-MG,PTH, AGEs, Hcy and IL-6 in the blood of the uremia patients is prepared by taking a mesoporous polystyrene hydrophobic resin as a carrier, which undergoes chloromethylation activation, and then performing chemical crosslinking grafting of vinylamine; and the adsorbent has large adsorption capacity, good mechanical strength, good biocompatibility and high adsorption selectivity. The adsorbent is used for treating uremia by blood perfusion, can take the advantages of blood filtering mainly for clearing micromolecular toxin, and provides a new method for treating uremia by combination of hematodialysis and blood perfusion.
Owner:山东卓逸医疗科技股份有限公司
High-sensitive blood-plasma total homocysteine detection reagent box
InactiveCN1979155AStrong detection specificityHigh sensitivityComponent separationOther chemical processesTotal homocysteineHomocysteine testing
The invention relates to a high sensitive blood plasma homocysteine testing kit that includes sample tube with stabilizer, reference substance and quality control serum of different Hcy thickness, DL- homocystine-D8 internal standard solution, reducer and trichloroacetic acid albumen precipitation. The invention has strong specificity for sample testing, and high sensitivity. It could be accurately and rapidly used in Hcy research.
Owner:上海特敏生物医药科技有限公司
Hcy detecting method and detecting kit
ActiveCN103926248AFast measurementImprove accuracyAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorPhysical chemistryBiology
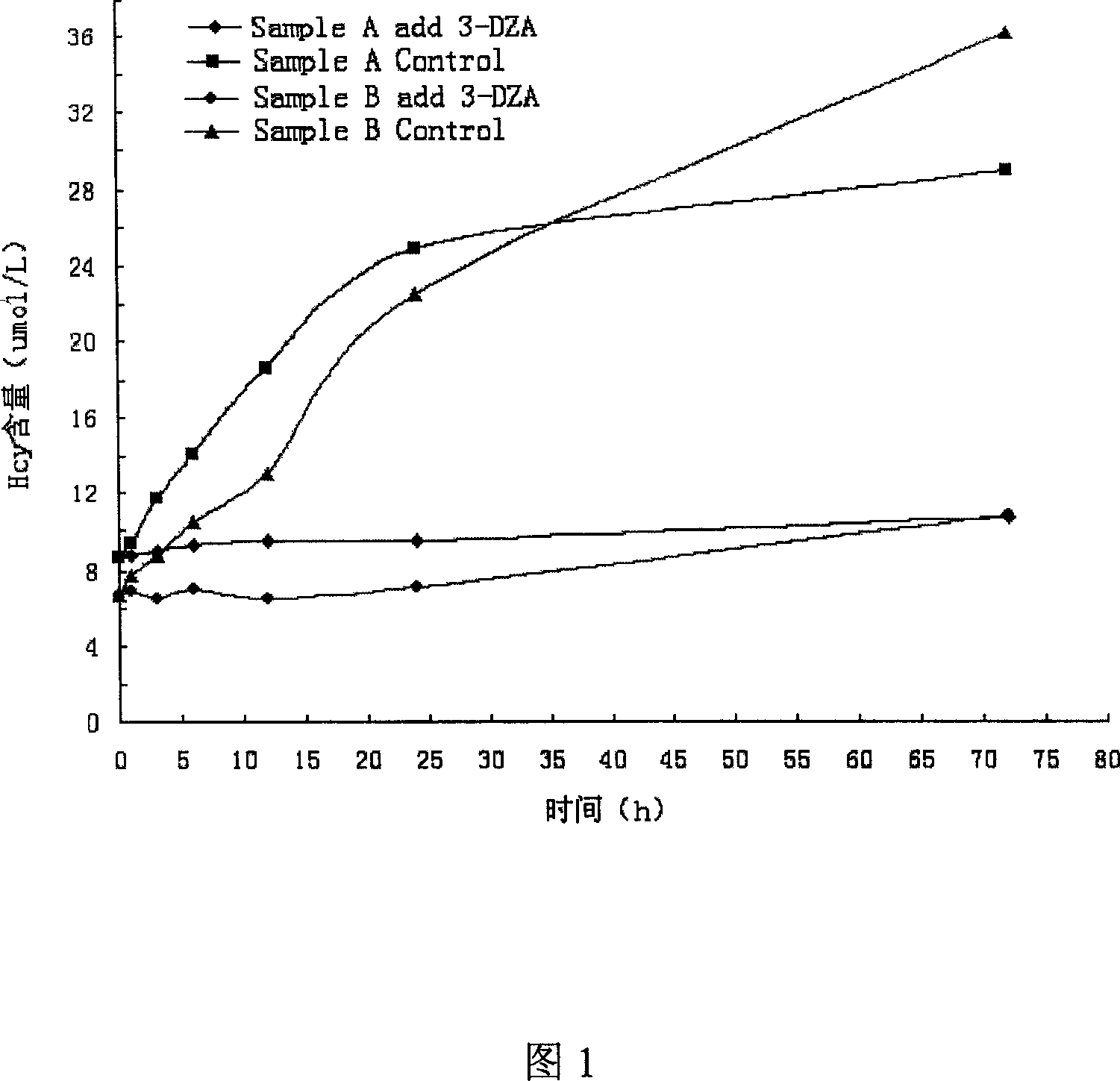
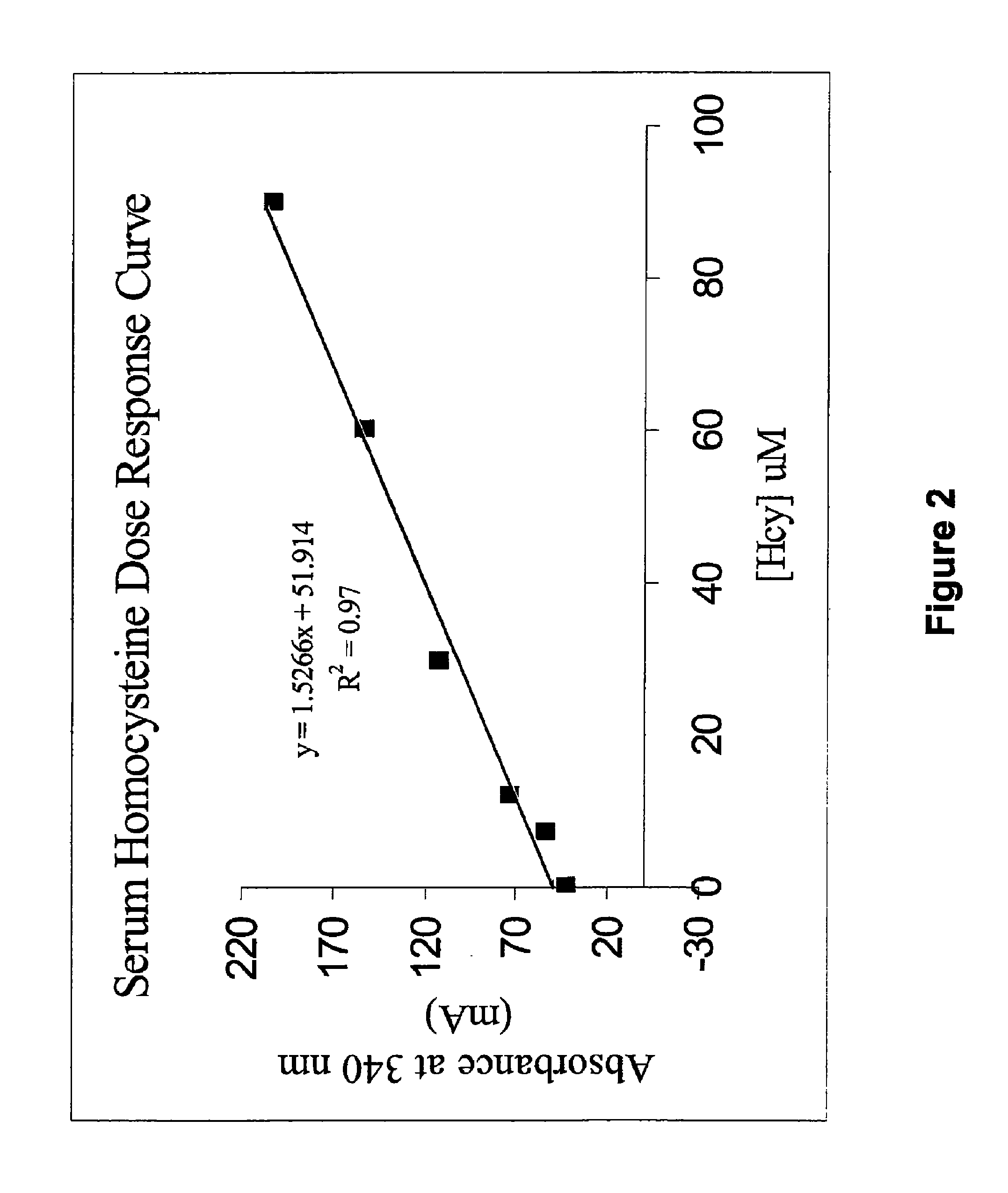
The invention provides an Hcy concentration detecting method. According to the principle, oxidized form Hcy is changed into free Hcy, the free Hcy reacts with serine under the catalysis of CBS to generate L-cystathionine, and then the L-cystathionine generates Hcy, pyruvic acid and NH3 under the catalysis of CBL. The pyruvic acid generated through circular reaction can be detected through LDH and NADH, the rate of the NADH which is changed into NAD is in direct proportion to the content of the Hcy in a sample, and the content of the Hcy can be detected by detecting the lowering rate of NADH+ at the position of 340 nm. The invention further provides a high-sensitivity Hcy concentration detecting kit.
Owner:浙江夸克生物科技有限公司
Glycine betaine and its use
InactiveUS20040033223A1BiocideSalicyclic acid active ingredientsBlood DisorderCystathionine synthase deficiency
Owner:MESSADEK JALLAL
High-efficiency non-integrated human iPSC induction platform
ActiveCN104673741AImprove induction efficiencyEasy to separateVertebrate cellsArtificial cell constructsAdenosineSomatic cell
The invention discloses a high-efficiency non-integrated human iPSC induction platform which comprises a composition, wherein the composition is used for inducing a human cell into iPSC, and the composition comprises a previous inducer and a later inducer; the previous inducer comprises the following active components: a transforming growth factor beta inhibitor, a glycogen synthetase kinase 3 inhibitor, a cAMP agonist, an S-adenosyl homocysteine hydrolase inhibitor and a p21 activated kinase inhibitor; and the later inducer comprises the following active components: the glycogen synthetase kinase 3 inhibitor, a selective ATP noncompetitive MEK inhibitor and the S-adenosyl homocysteine hydrolase inhibitor. The high-efficiency non-integrated human iPSC induction platform disclosed by the invention achieves the previous induction efficiency of the iPSC of 6.4% under the action of a previous inducer composition, can achieve the induction of a full culture of 20.8% through later induction culture or be used for totally further inducing a previous inductor clone into a human iPSC clone approaching to ESC and is far higher than the prior art in induction efficiency; in addition, the obtained iPSC is high in maturity, free of being inserted with an exogenous gene, is more approaching to the ESC in cell morphology and property and has the advantages of good stability and great high application value.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
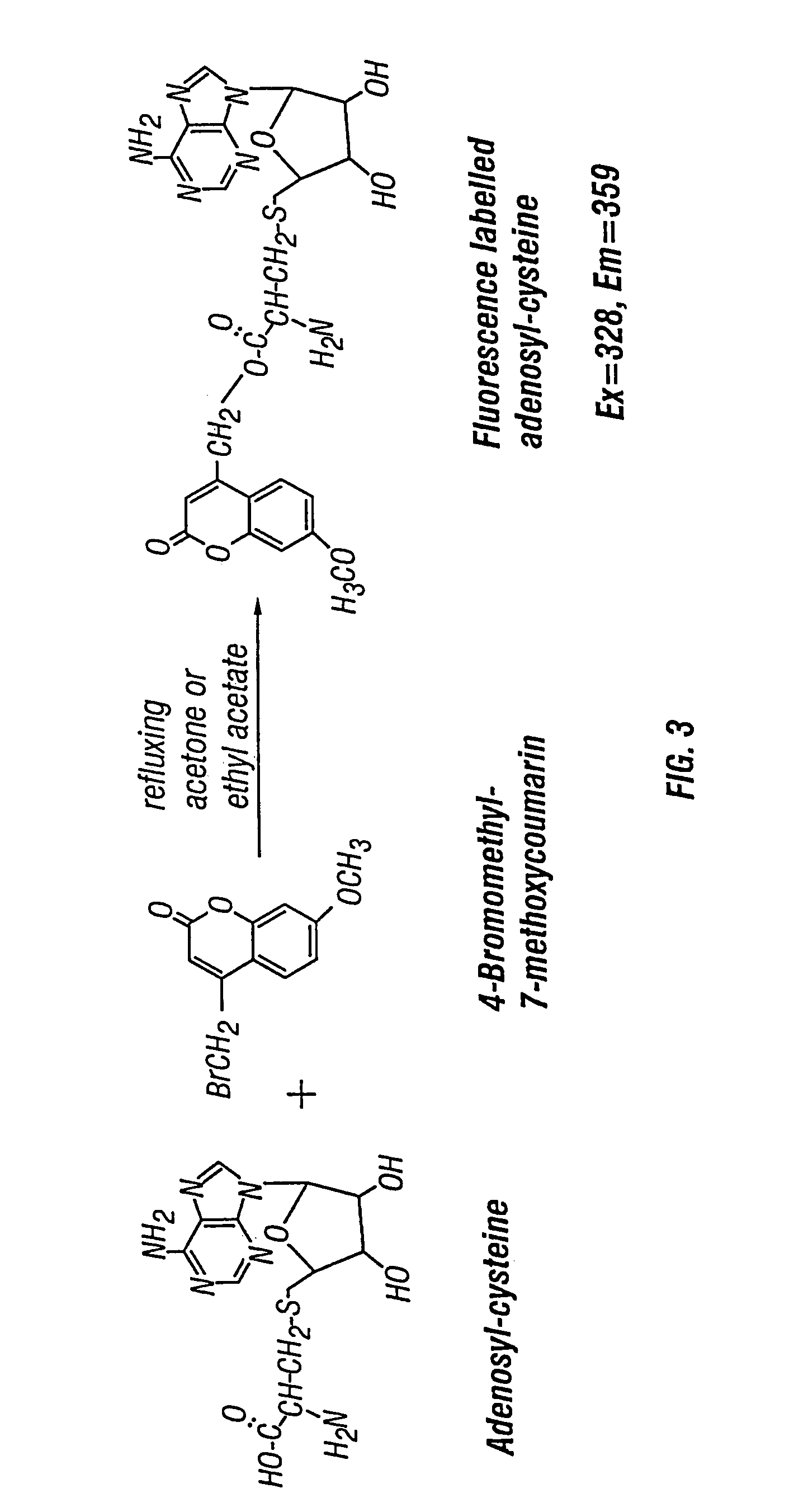
Use of fluorescence for the quick and easy determination of s-adenosylmethionine, s-adenosylhomocysteine and homocysteine
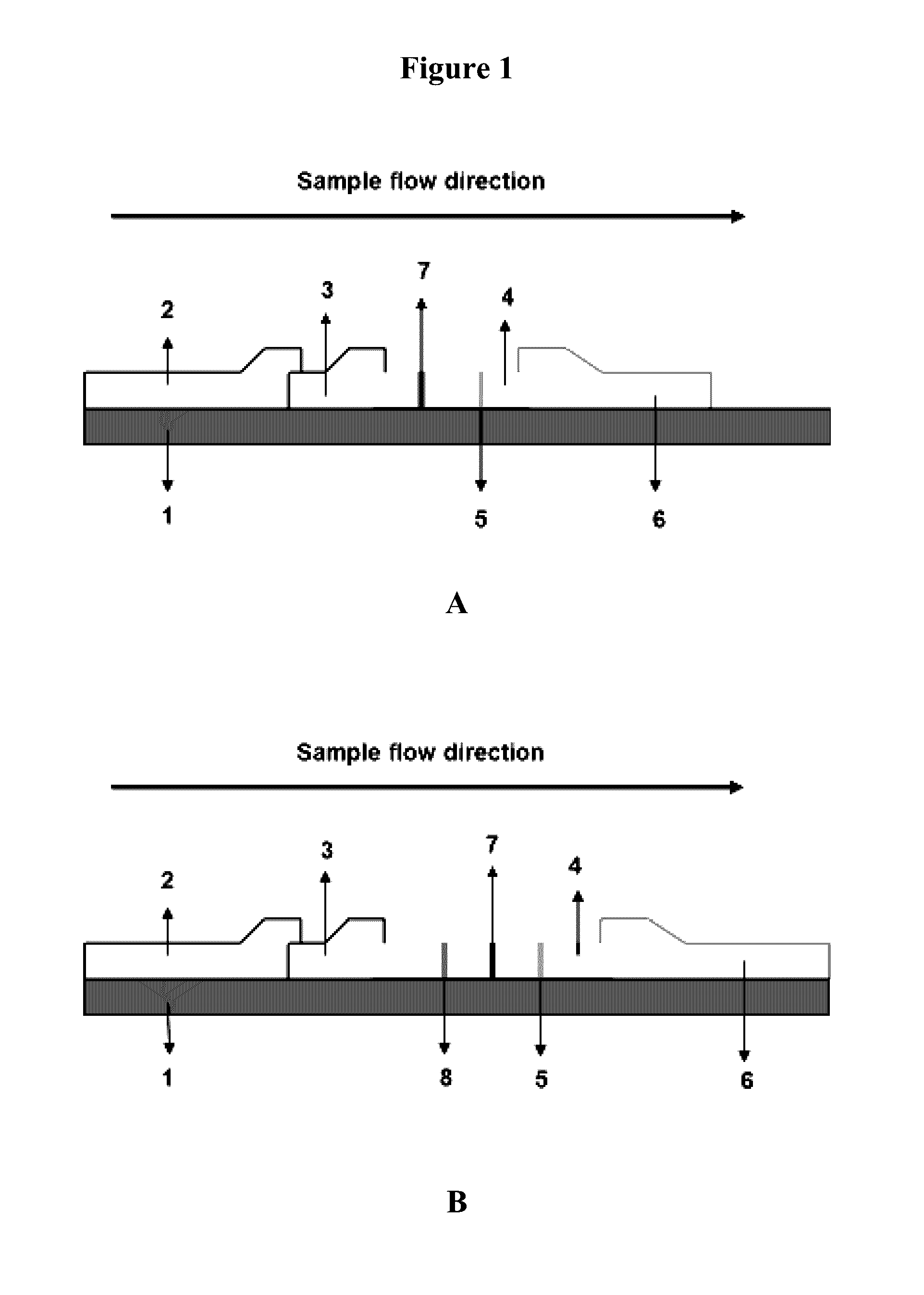
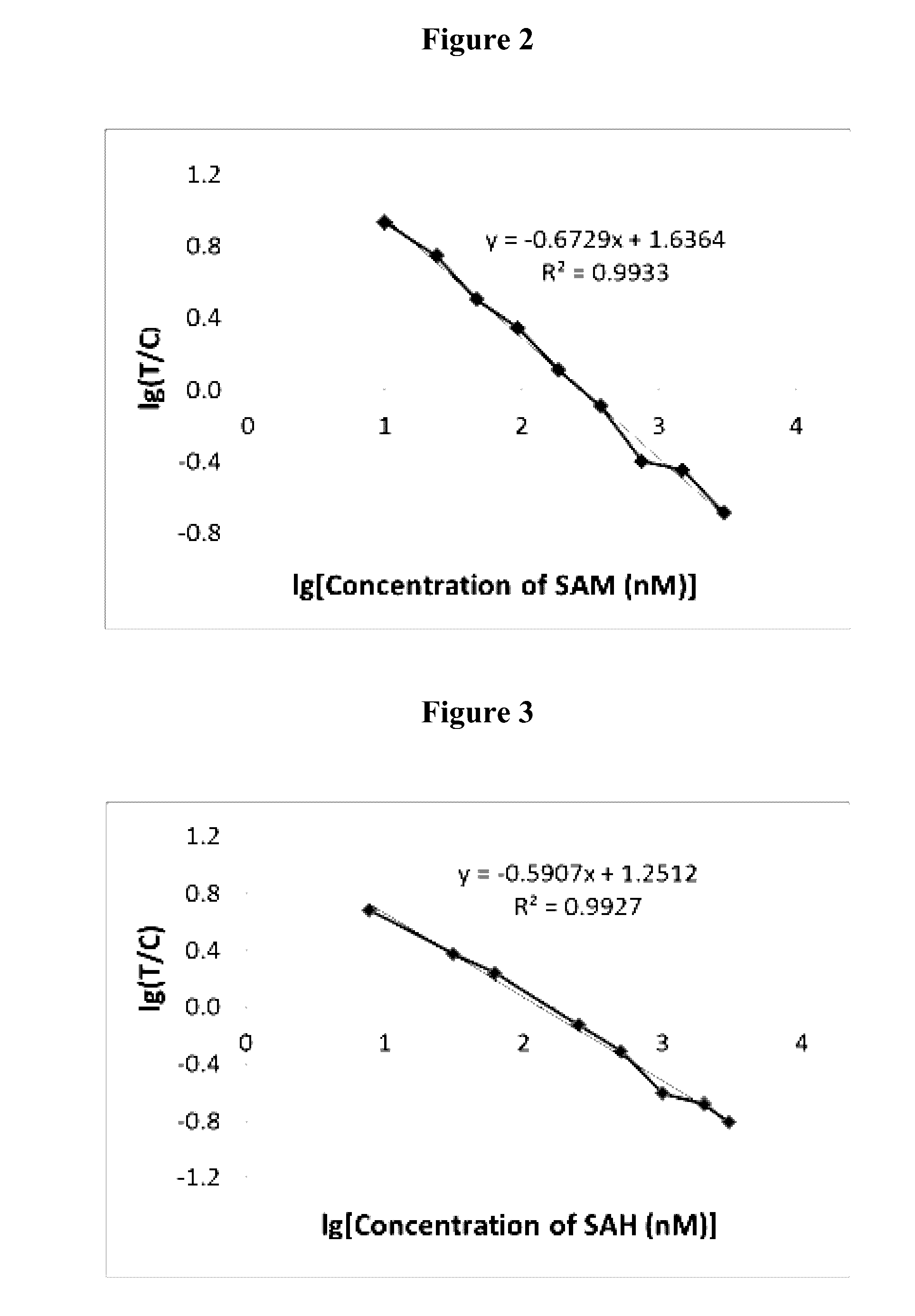
The invention provides immunochromatographic test strips and methods for detecting and quantifying S-Adenosylmethionine (SAM), S-Adenosylhomocysteine (SAH) and Homocysteine (HCy) in a sample, comprising: (a) making fluorophore conjugated antibodies; (b) immobilizing SAM, SAH and HCy on a solid support; (c) providing a sample, combining said sample with a conjugate selected from the group consisting of lanthanide chelate conjugates and quantum dot conjugates (QD) with anti-SAM, anti-SAH or anti-HCy, wherein said combining is performed under conditions that allow formation of a competitive complex comprising said conjugate, said SAM, SAH or HCy on the solid support and SAM, SAH or HCy in a sample when present; and (d) detecting the presence of the complex, if present, by monitoring a spectral emission mediated by the fluorescent conjugates in the complex, wherein the emission indicates the presence and quantity of SAM, SAH or HCy in the sample.
Owner:HUNAN SKYWORLD BIOTECH
Strong interference resistant homocysteine detection kit
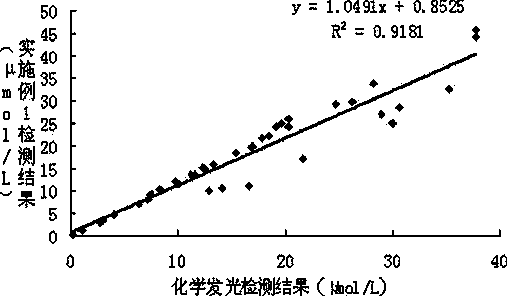
ActiveCN104111337AImprove anti-interference abilityHigh sensitivityBiological material analysisBiological testingHomocysteineChemiluminescence
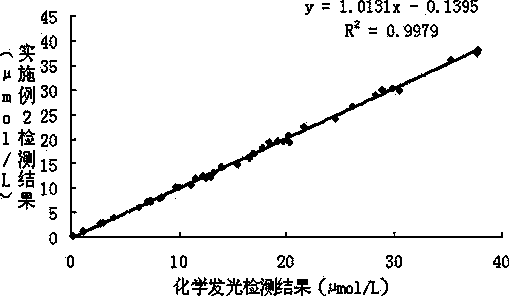
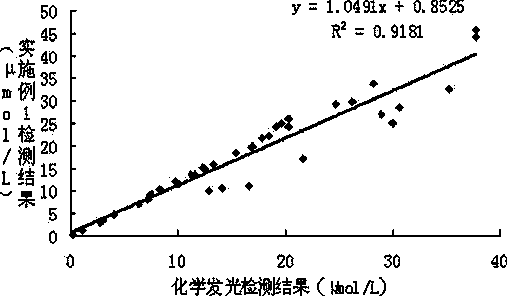
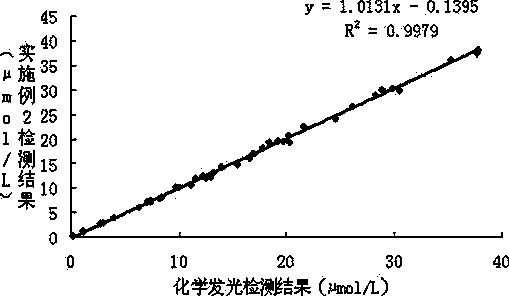
The invention provides a strong interference resistant homocysteine detection kit. The kit comprises a component 1 and a component 2 according to a volume ratio of 4:1. The result obtained by using the kit is highly consistent with the result obtained through chemiluminescent detection, the accuracy and the sensitivity of detected clinic samples of the kit are better than that of routine reagents, and it is in favor of improving the clinic detection accuracy of homocysteine.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Strong interference resistant homocysteine detection kit
ActiveCN104111338AImprove anti-interference abilityReduce detection impactBiological material analysisBiological testingOxidative enzymeHomocysteine
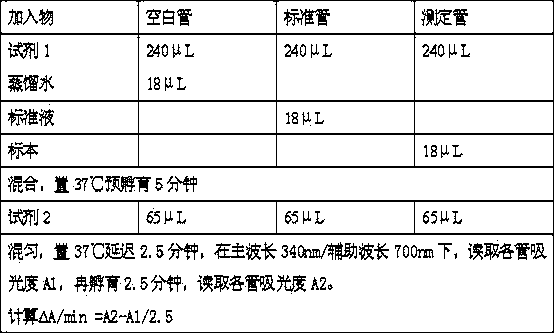
The invention provides a strong interference resistant homocysteine detection kit. The kit comprises a component 1 and a component 2 according to a volume ratio of 240:65. The addition of ascorbic acid oxidase to the component 1 effectively enhances the interference resistance and reduces the influences of pyruvic acid and interference substances on the detection of the kit in order to enhance the reagent detection accuracy; the detection result of the kit provided in the invention is highly consistent with the detection result of a chemiluminescent detection kit; and the strong interference resistant homocysteine detection kit has higher sensitivity and accuracy than routine homocysteine detection kits, enhances the market competitiveness, and is in favor of the reagent market popularization.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
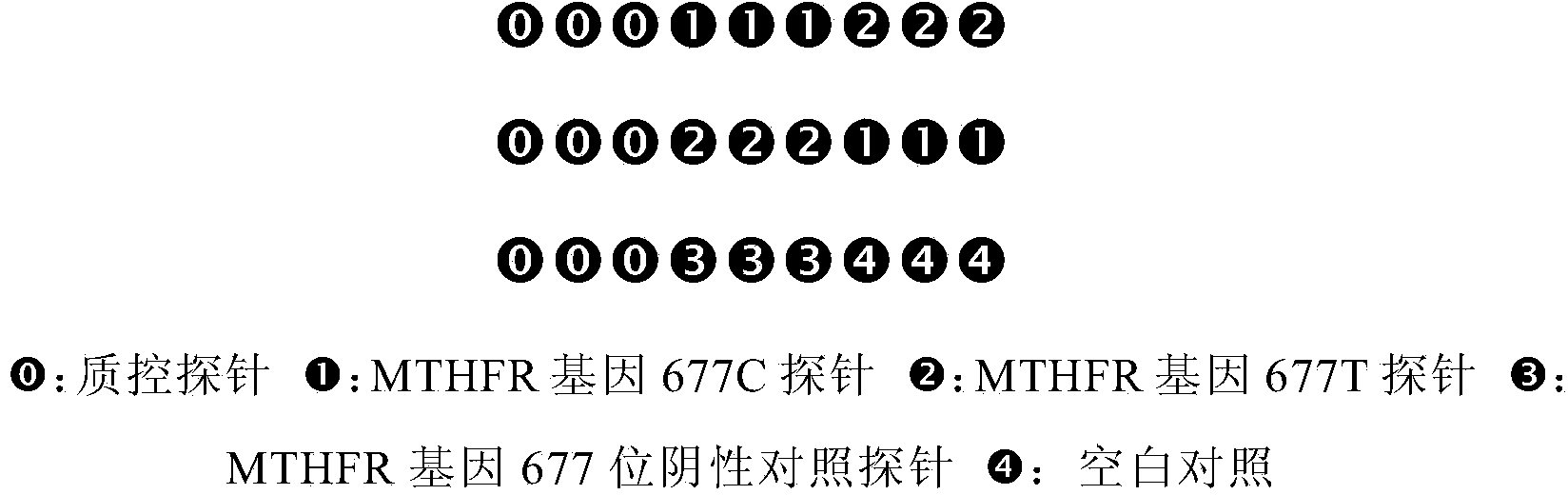
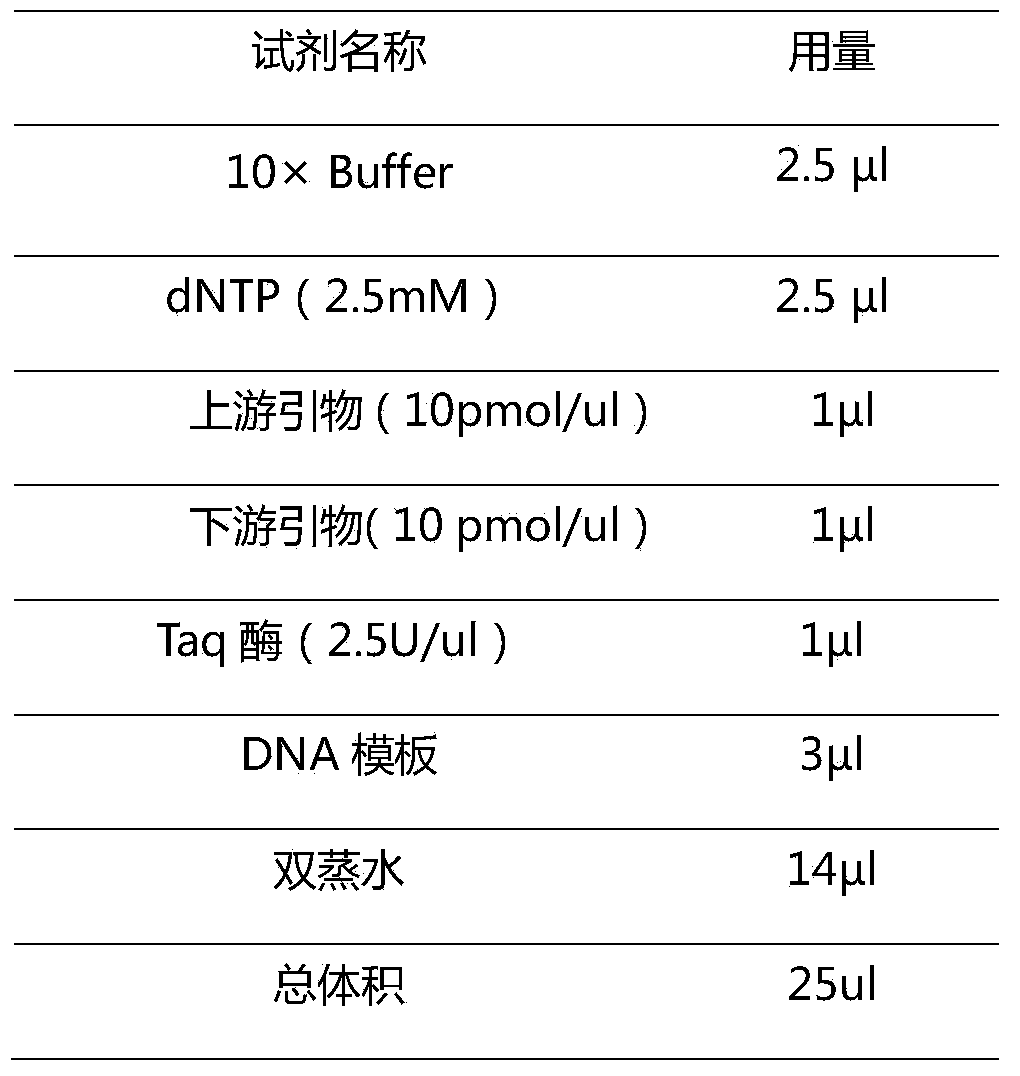
Pair of specific primers and probe for detection of MTHFR gene chip
InactiveCN103525924AGood signal to noise ratioStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotideCysteine
The invention relates to the molecular biology field, and discloses a specific oligonucleotide probe for detection of an SNP locus of rs1801133677C / T of an MTHFR gene and a pair of specific primers for amplification of a target zone containing the target locus to be detected during detection of the above SNP locus. The oligonucleotide probe can hybridize with different genotypes of the 677th locus of the MTHFR gene specifically. The pair of specific primers can amplify a target zone containing the target locus to be detected. By utilization of a chip hybridization method, the probe and the above amplified zone are hybridized for detection of the MTHFR genotypes, which provides references for evaluation of suspicious patients with thrombophilia, increase of homocysteine level and change of folic acid metabolism, and provides information for doctors for correct selection of medicines and reasonable adjustment of medicine dosage.
Owner:SHANGHAI BAIO TECH
Method for detecting content of homocysteine in blood or urine
ActiveCN102565252AHigh recovery rateAccurate identificationComponent separationMatrix solutionChromatography column
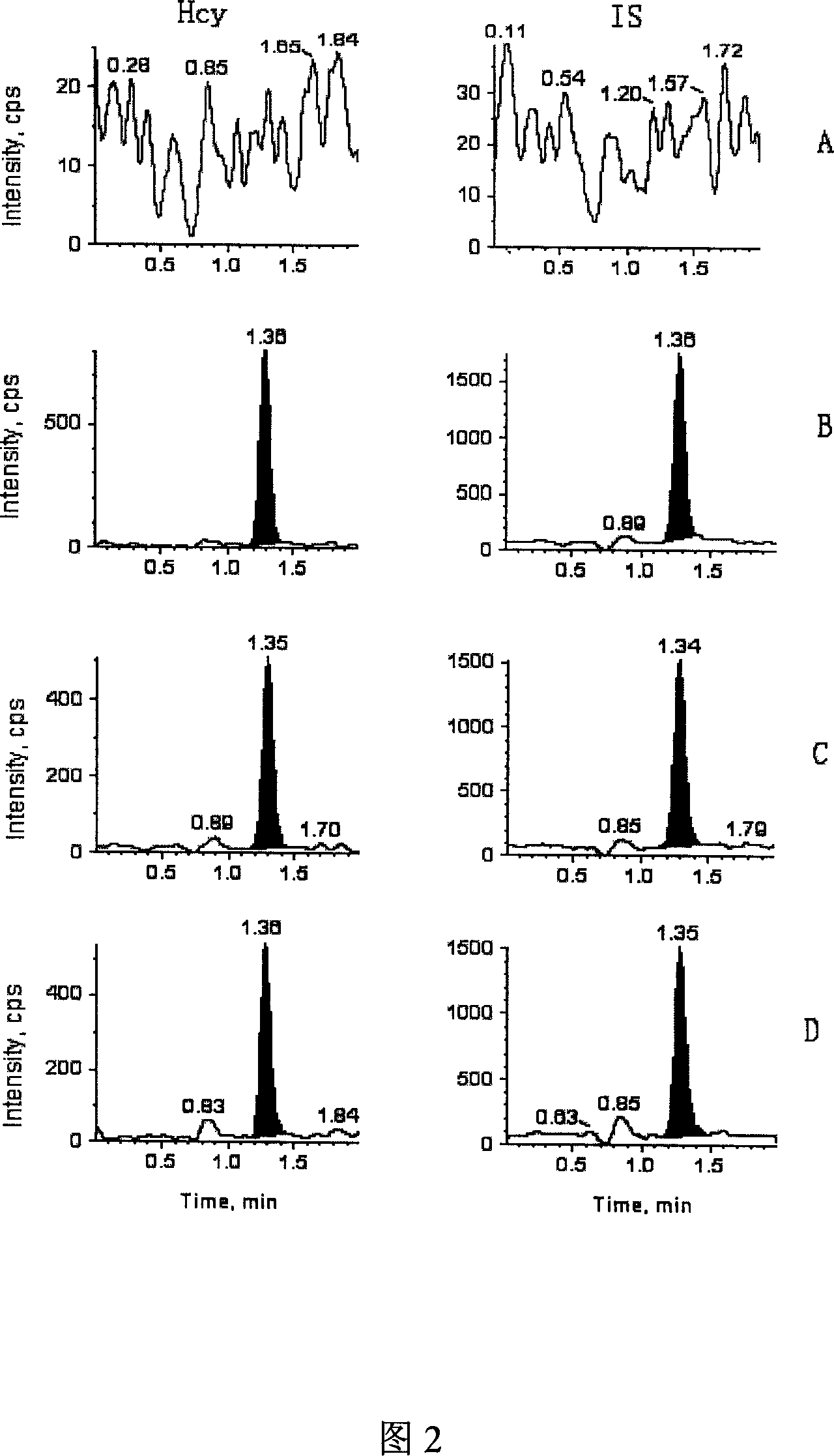
The invention discloses a method for detecting the content of homocysteine in blood or urine. The method comprises the following steps: 1, respectively mixing a standard substance and a sample to be tested with an internal standard solution, adding a matrix solution, and uniformly mixing; 2, adding mercaptoethanol, dithiothreitol or TCEP (trichloroethyl phosphate), uniformly mixing, and allowing the obtained solutions to stand for 10min; 3, adding trichloroacetic acid with the mass percentage of 10%, centrifuging at a high speed, taking the obtained supernatants, diluting the supernatants by deionized water, carrying out liquid phase-mass spectrometry by respectively allowing the diluted supernatants and a mobile phase to flow through a chromatographic column, and finally determining the content of the homocysteine in the blood or the urine. The method for detecting the content of the homocysteine in the blood or the urine of the invention has the advantages of simplicity, rapidness and sensitivity, can be applied to the screening of a large amount of samples, and makes accurate quantification and strong specificity be realized.
Owner:郑州和合医学检验实验室有限公司
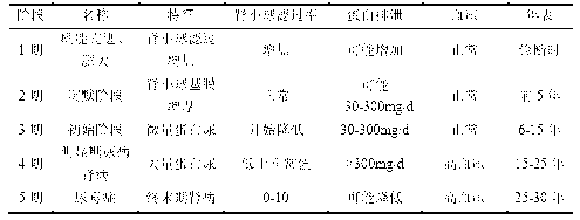
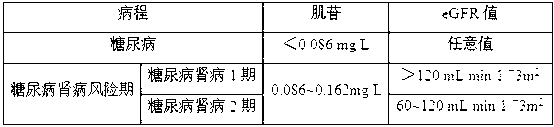
Diabetic nephropathy diagnostic kit and application thereof
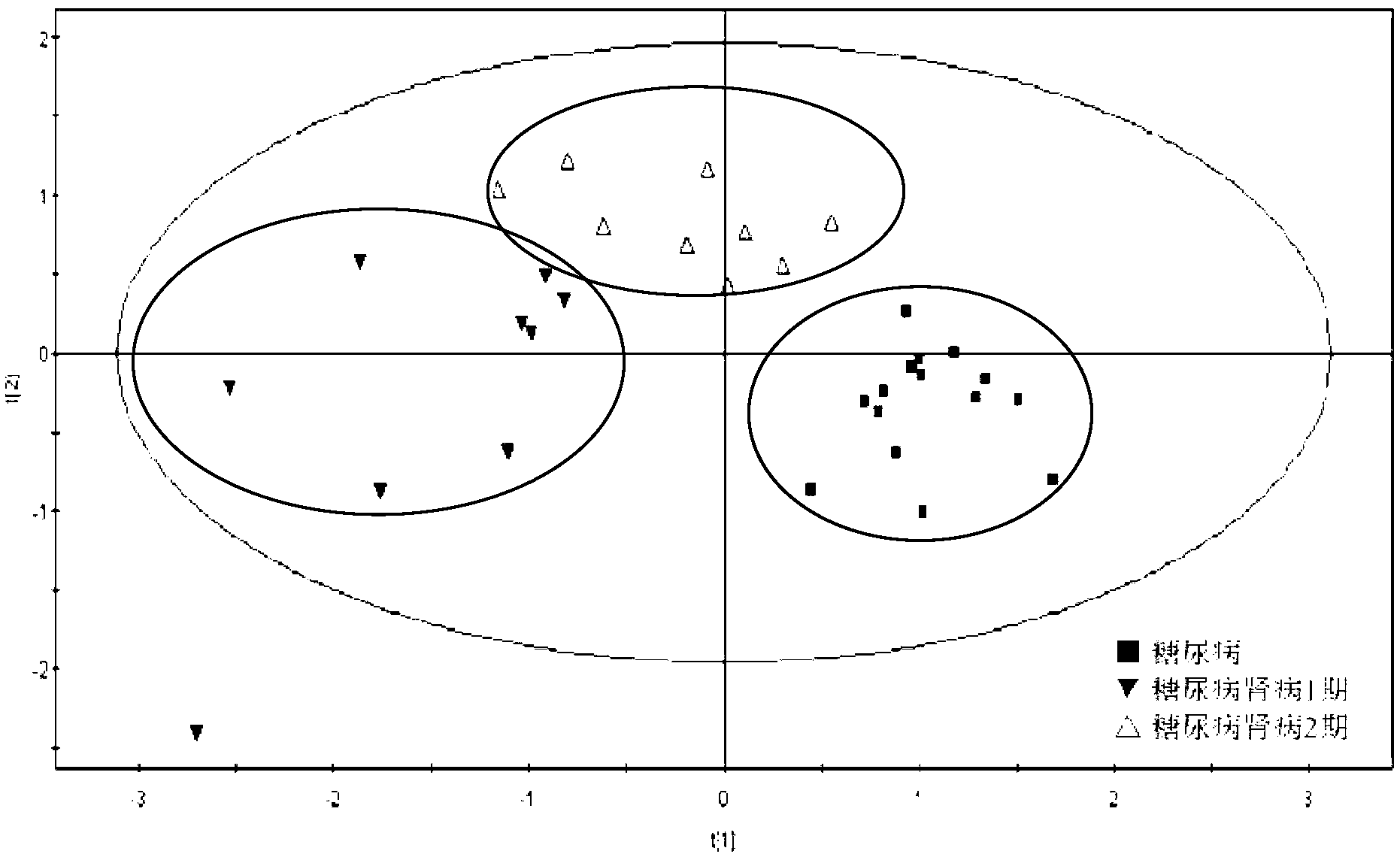
The invention discloses a diabetic nephropathy diagnostic kit and the application thereof, and particularly discloses the application of inosine serving as a biomarker in preparation of a diagnostic kit for diabetes and the diabetic nephropathy, and the application of the combination of the inosine, adenosine and S-adenosyl homocysteine and the combination of the adenosine, the S-adenosyl homocysteine and linoleic acid serving as markers in preparation of the diabetic nephropathy diagnostic kit. The invention also discloses the diagnostic kit prepared by the biomarkers, which can be used for distinguishing the diabetic nephropathy and staging different development stages of the diabetic nephropathy, performing screening and assessment analysis on the treatment medicaments for different development stages of the diseases, and provides accurate basis for the treatment and the diagnosis of the diabetes and the diabetic nephropathy.
Owner:罗国安
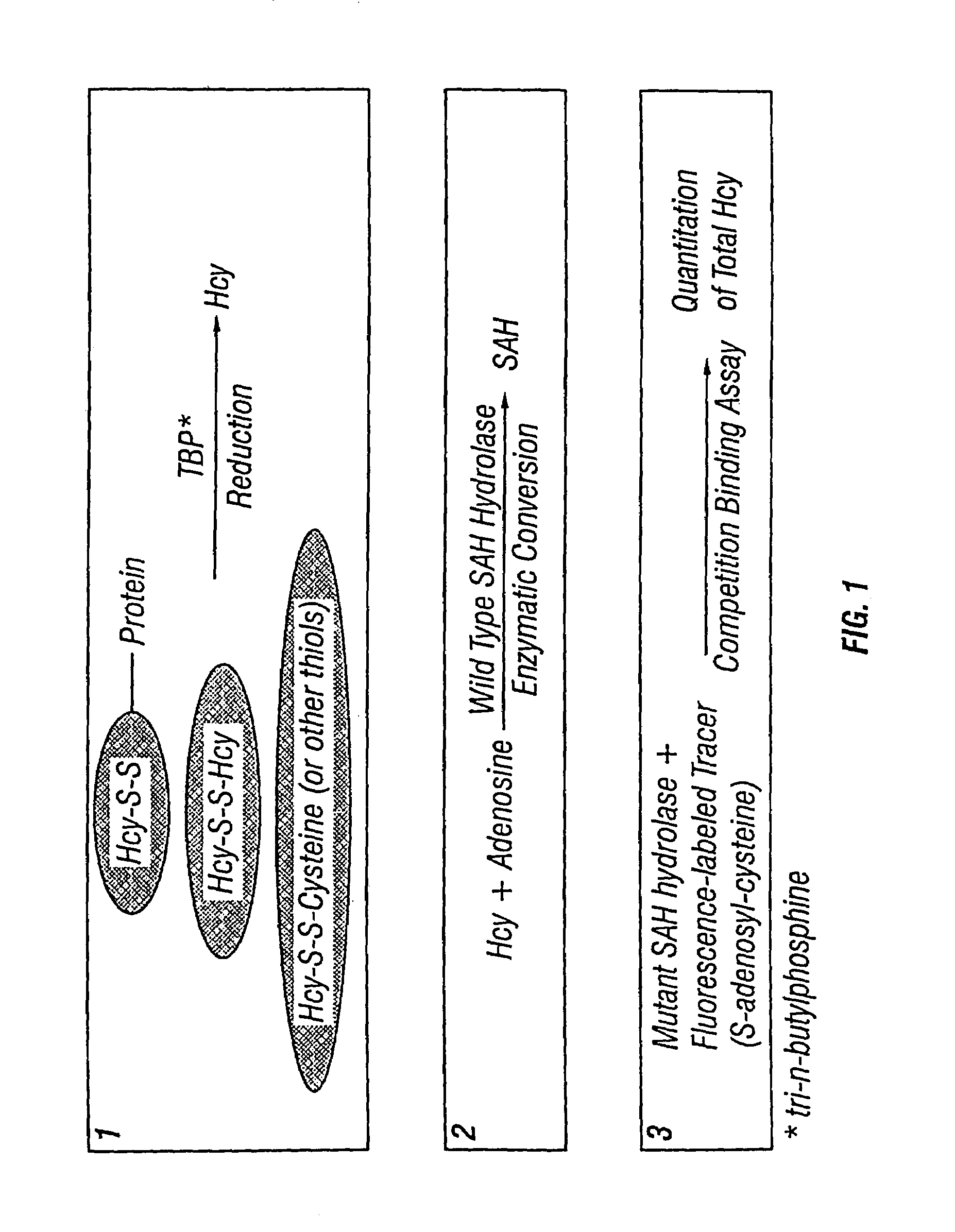
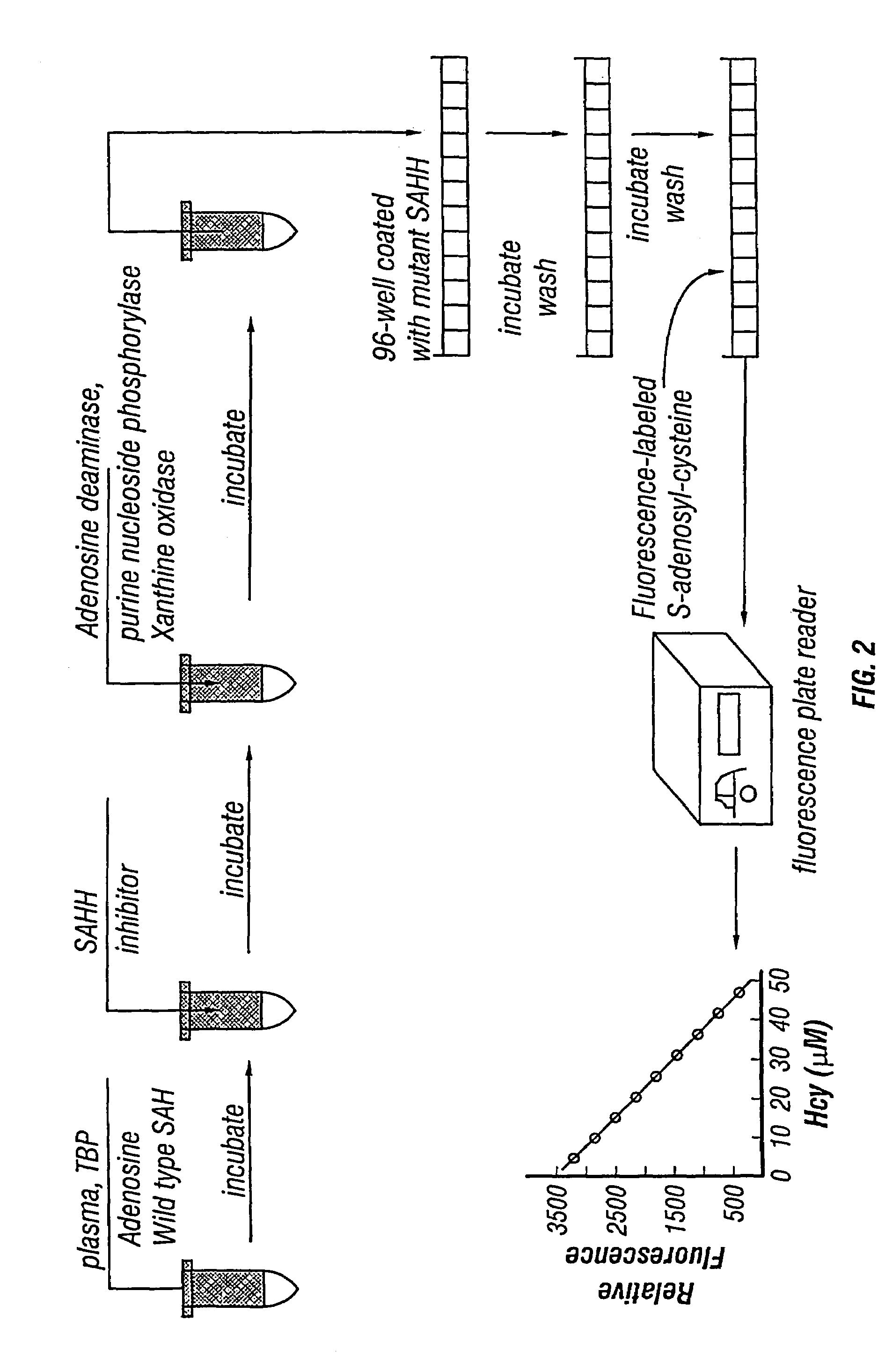
Methods for assaying homocysteine
InactiveUS7192729B2Significant comprehensive benefitsHigh detection sensitivityHydrolasesMicrobiological testing/measurementAdenosineMoiety
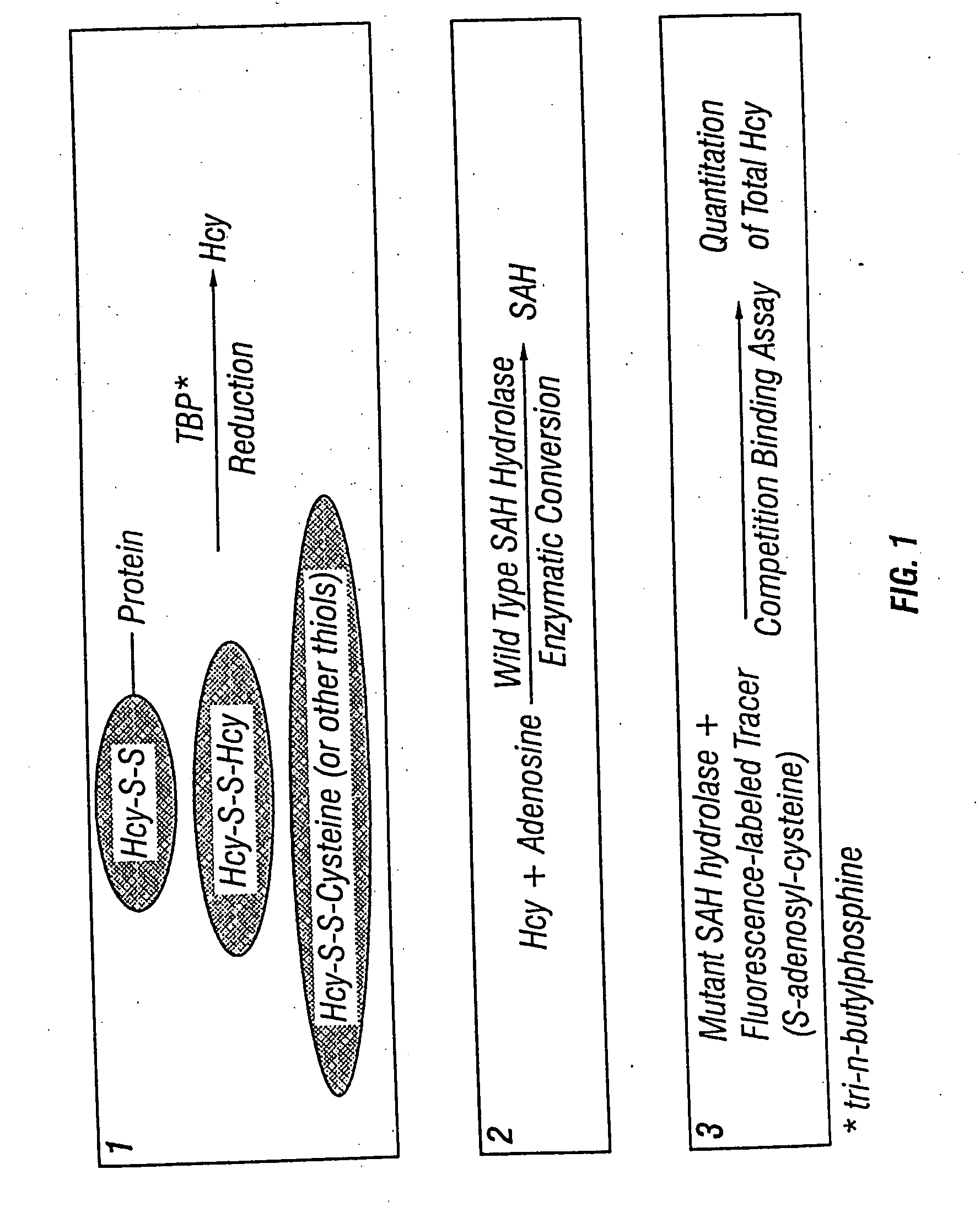
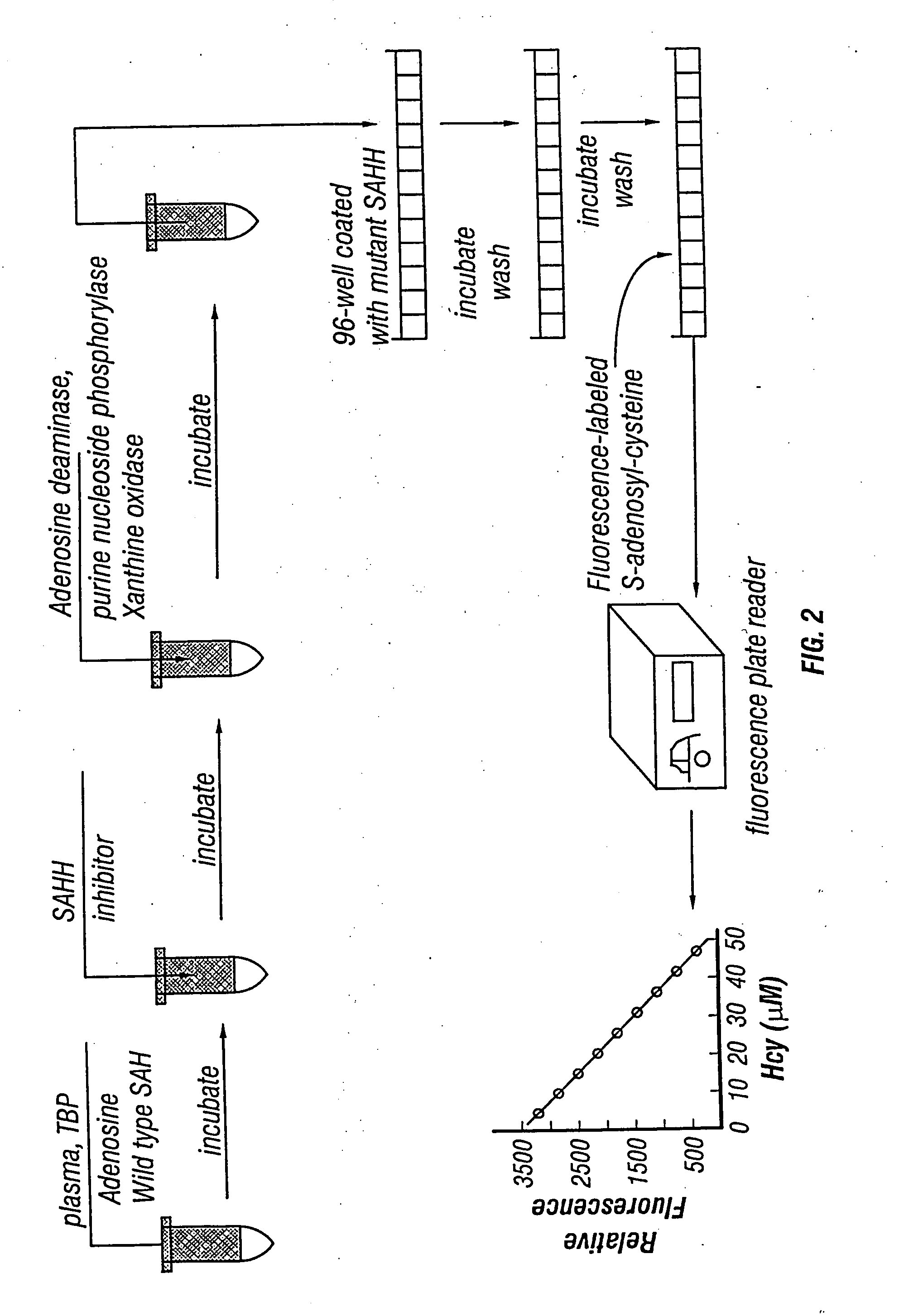
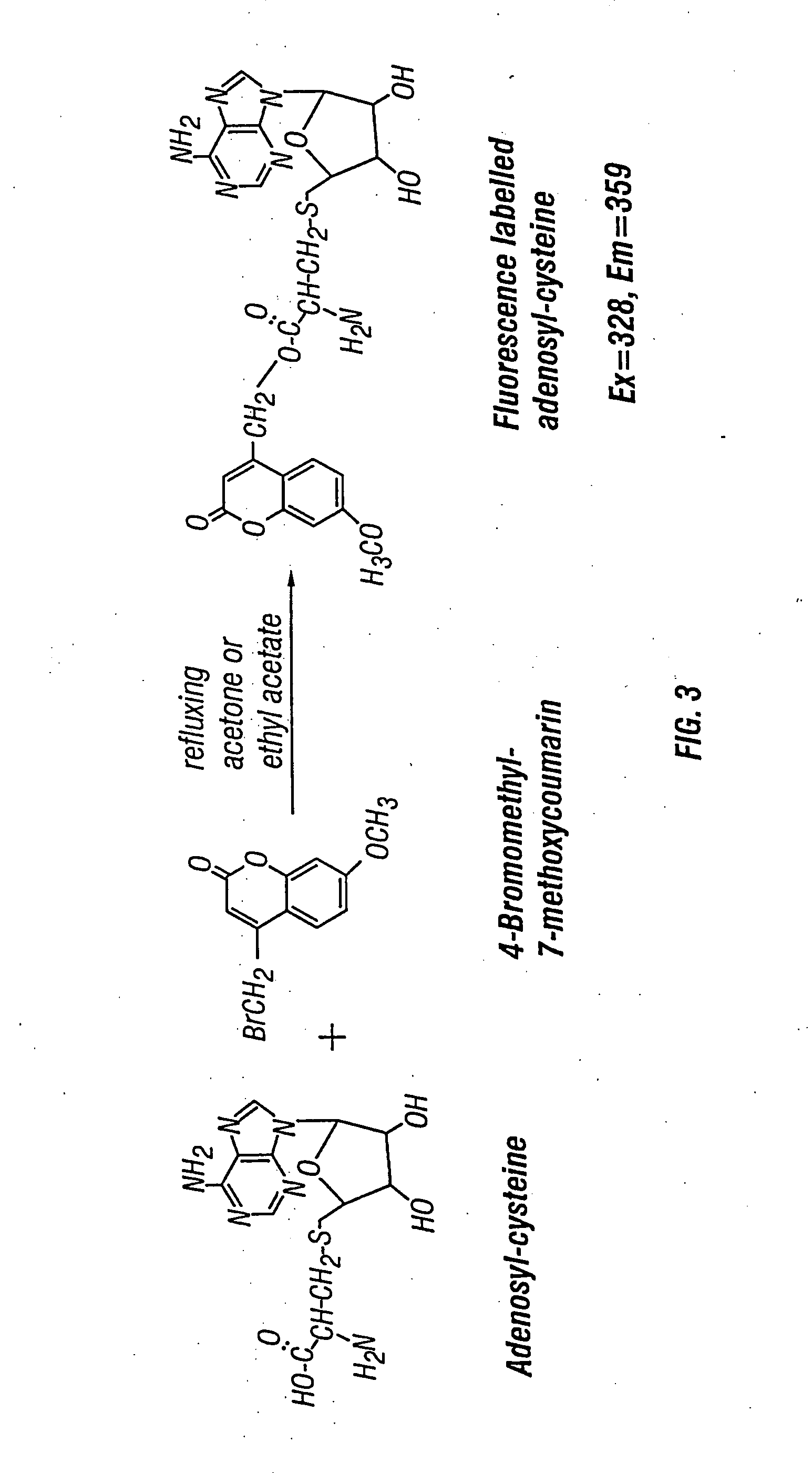
The present invention relates to compositions and methods for assaying homocysteine (Hcy) and thus related moieties, e.g., S-adenosylhomocysteine (SAH) or adenosine. More particularly, assay methods that employ, mutant SAH hydrolase having binding affinity for Hcy, SAH or adenosine but has attenuated catalytic activity, are provided. The modified enzymes and fusion proteins containing the modified enzymes are also provided.
Owner:DIAZYME LAB INC
Method for simultaneously detecting homocysteine and metabolism-related substances thereof
PendingCN112834677AEasy to handleReliable resultsComponent separationPyridoxineTandem mass spectrometry
The invention relates to a method for simultaneously detecting homocysteine and metabolism-related substances thereof. The method comprises the following steps: pre-treating a blood sample, analyzing and detecting by adopting liquid chromatography-tandem mass spectrometry, and finally quantifying by adopting an internal standard method. The method can be used for simultaneously detecting the contents of homocysteine and metabolism-related substances (methionine, pyridoxine, folic acid and 5-methyltetrahydrofolic acid) in human serum, and carrying out accurate qualitative and quantitative analysis, and is a detection method which is simple in sample treatment, high in flux and reliable in result.
Owner:质谱生物科技有限公司
Methods and compositions for assaying homocysteine
InactiveUS20060246529A1High detection sensitivitySignificant comprehensive benefitsHydrolasesMicrobiological testing/measurementAdenosineMoiety
The present invention relates to compositions and methods for assaying homocysteine (Hcy) and thus related moieties, e.g., S-adenosylhomocysteine (SAH) or adenosine. More particularly, assay methods that employ, mutant SAH hydrolase having binding affinity for Hcy, SAH or adenosine but has attenuated catalytic activity, are provided. The modified enzymes and fusion proteins containing the modified enzymes are also provided.
Owner:DIAZYME LAB INC
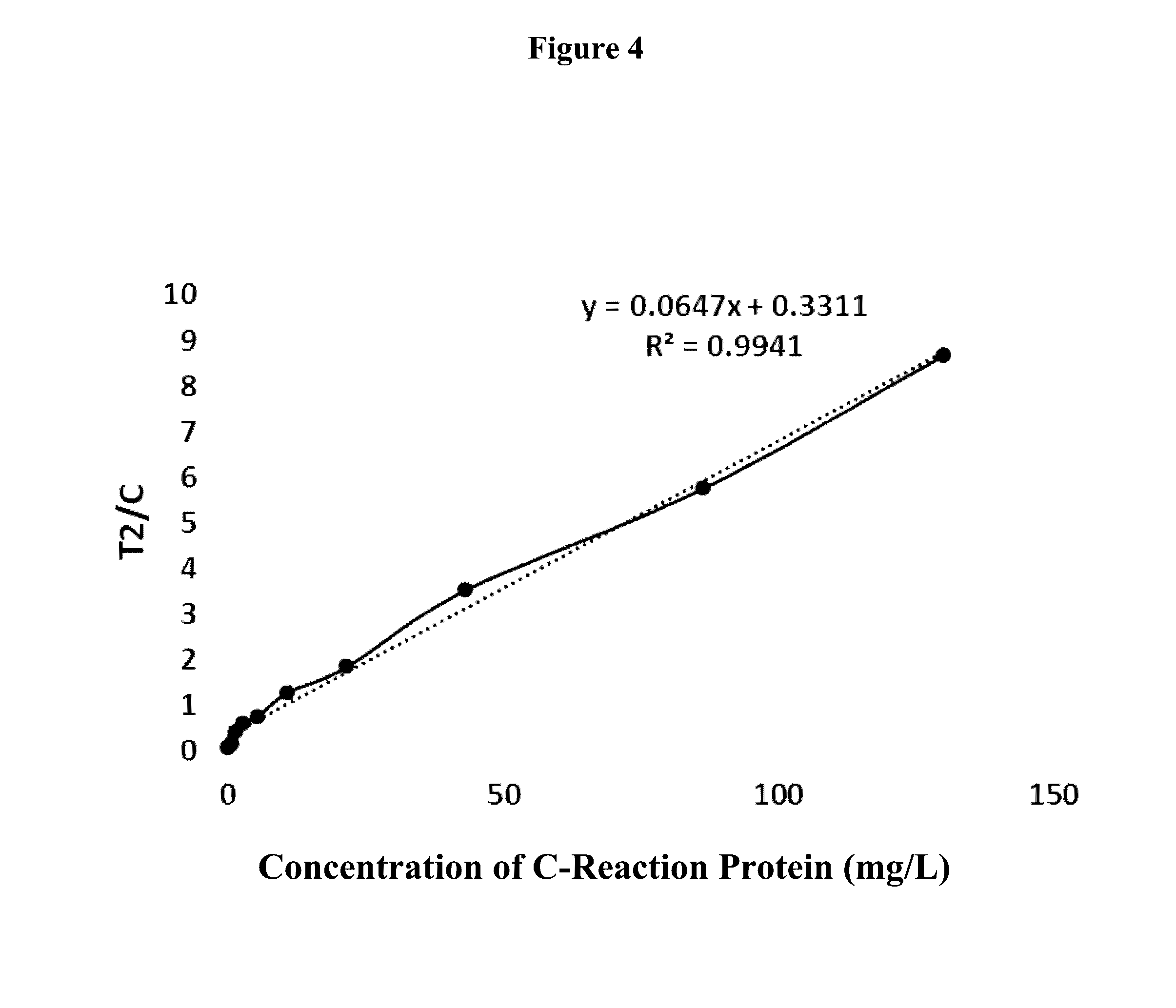
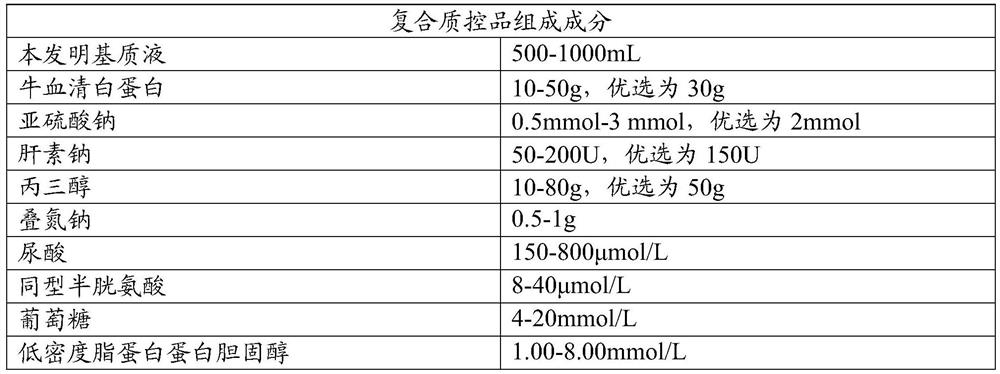
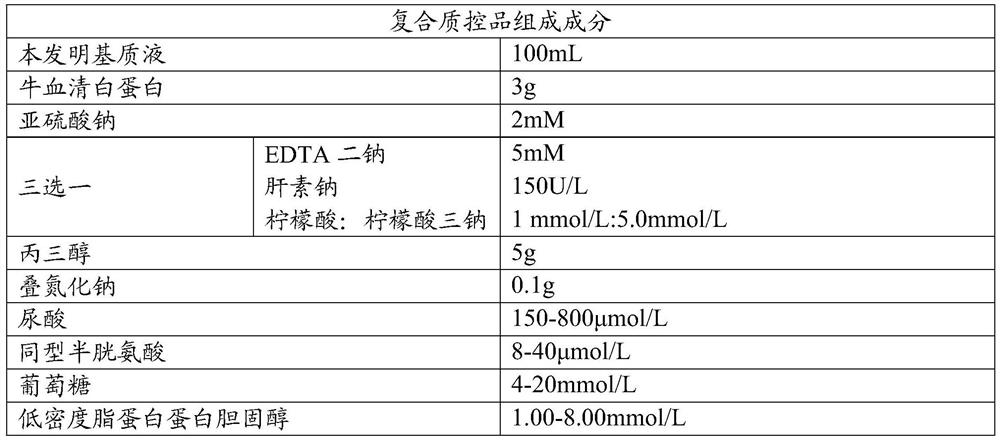
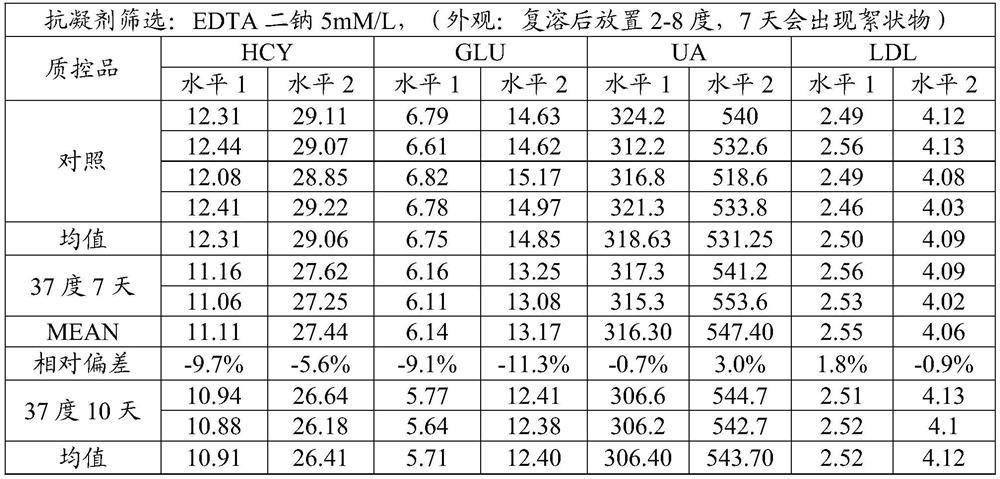
Cardiovascular and cerebrovascular and diabetes related four-high index composite quality control product and preparation method thereof
ActiveCN112180095APromote formationEnsure consistencyDisease diagnosisBiological testingAnticoagulant AgentA lipoprotein
The invention relates to the technical field of medical diagnosis, and discloses a cardiovascular and cerebrovascular and diabetes related four-high index composite quality control product and a preparation method thereof. The composite quality control product comprises a matrix liquid, a reducing agent, bovine serum albumin, an anticoagulant, polyol, uric acid, homocysteine, glucose, low-densitylipoprotein cholesterol and a preservative. The composite quality control product which can be used for testing four cardiovascular and cerebrovascular and diabetic indexes of Glu, Hcy, UA and LDL-C at the same time can be prepared at a time, healthy human plasma is selected as a matrix liquid raw material, the components are basically consistent with those of serum after treatment, and the matrixeffect is reduced to the maximum extent; meanwhile, by matching with other components, the prepared four-high quality control product has good uniformity and stability, stable performance, long preservation time and simplicity and convenience in use, meanwhile, the accuracy of quality control during joint inspection is greatly improved, and a more accurate basis is provided for clinical diagnosisof cardiovascular and cerebrovascular diseases and diabetes related diseases.
Owner:SINOCARE
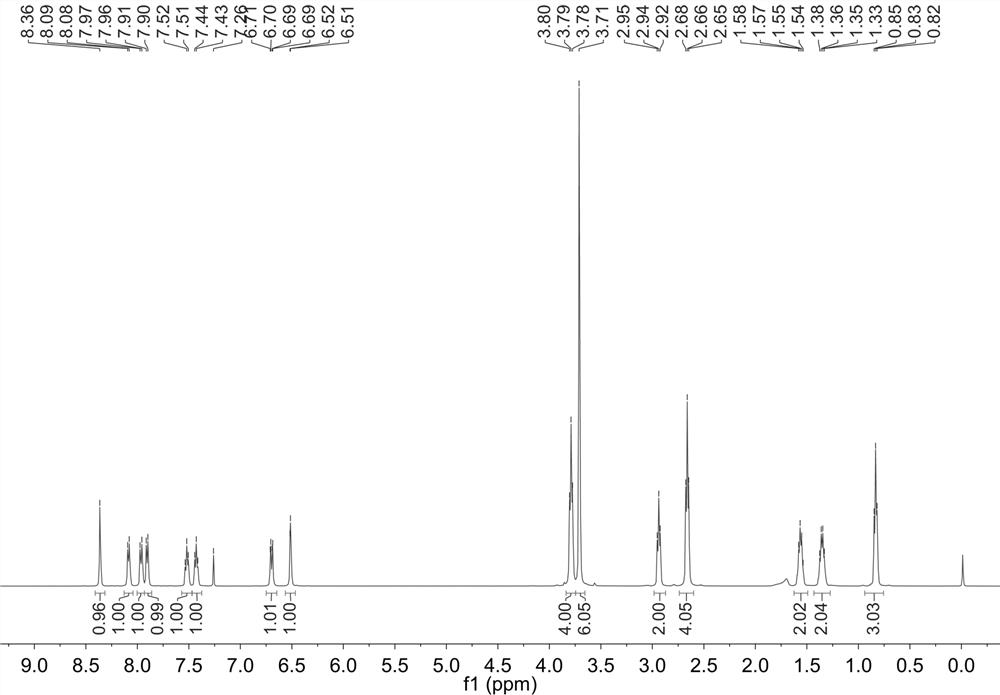
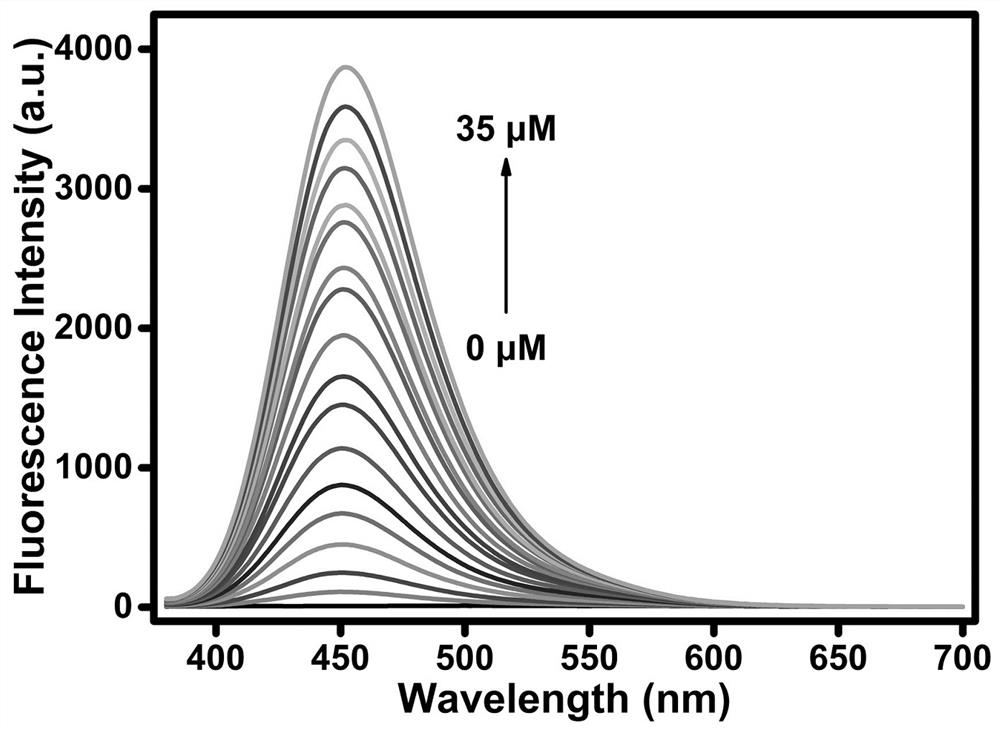
Synthesis of multi-signal fluorescent probe and application of multi-signal fluorescent probe in detection of Cys, GSH and Hcy
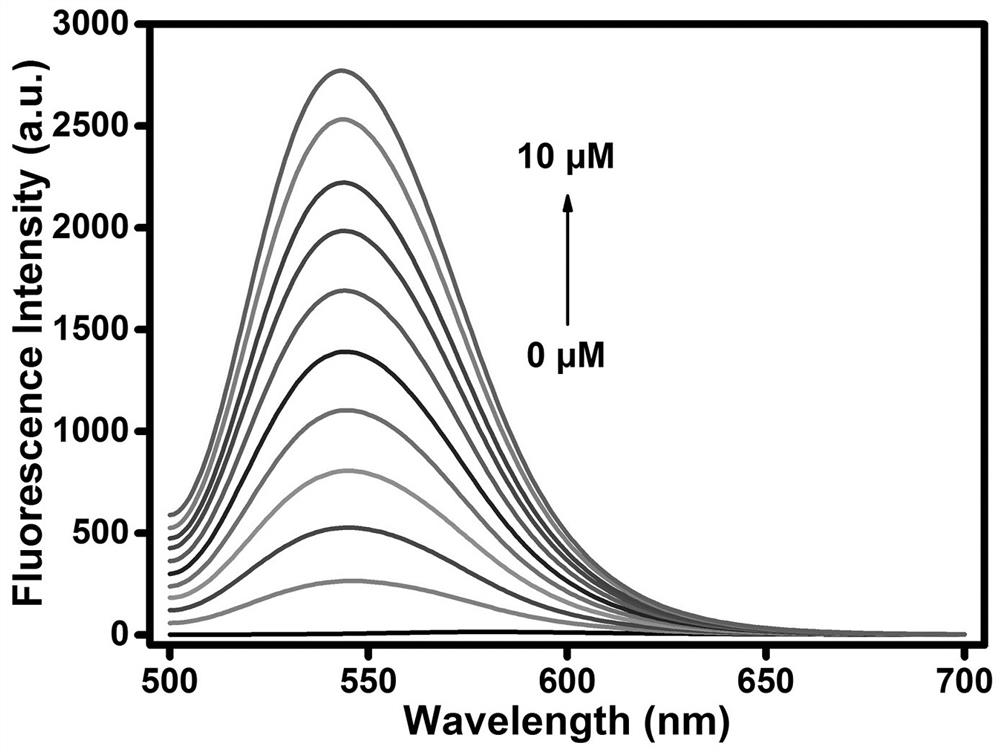
The invention discloses synthesis of a multi-signal fluorescent probe and an application method of the multi-signal fluorescent probe in quantifying serum total cysteine (Cys) and homocysteine (Hcy) and simultaneously distinguishing Cys, glutathione (GSH) and Hcy in fluorescence imaging cells / living bodies. The chemical structural formula of the molecular probe is shown in the specification. The fluorescent probe disclosed by the invention has a plurality of reaction sites with controllable activity, and can be used for respectively detecting Cys (lambdaex / lambdaem = 360 / 453 nm), GSH (lambdaex / lambdaem = 415 / 513 nm) and Hcy (lambdaex / lambdaem = 488 / 542 nm) through three channels of blue, green and yellow. The fluorescent probe has the advantages of good selectivity, high sensitivity and strong anti-interference performance, and can be used for directly and simultaneously quantifying the total Cys and Hcy levels in plasma and simultaneously distinguishing Cys, GSH and Hcy in fluorescence imaging cells / living bodies.
Owner:HUNAN NORMAL UNIVERSITY
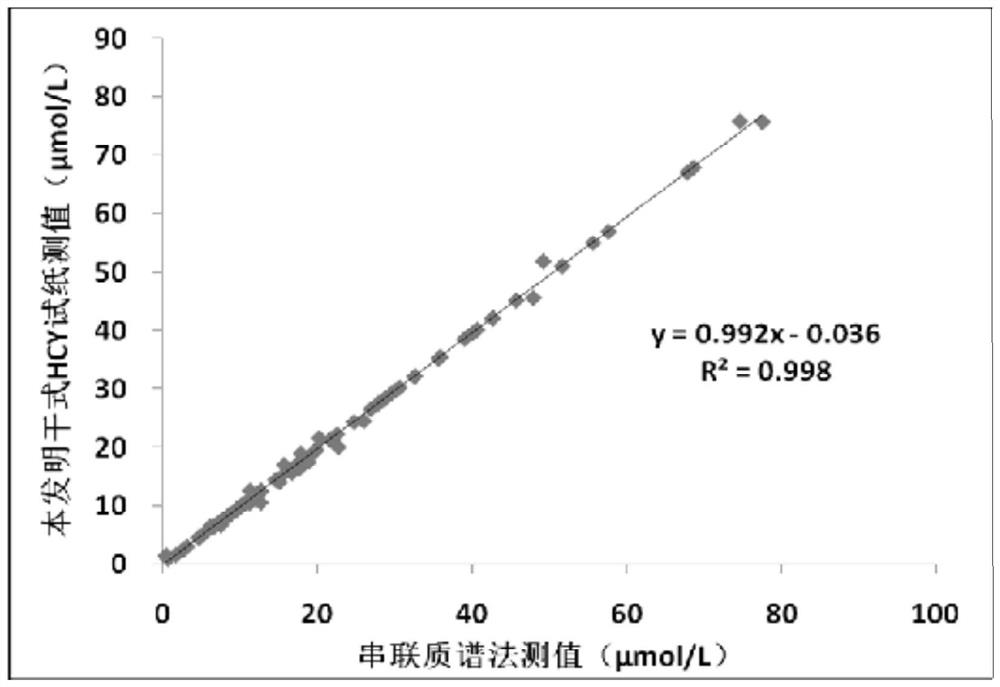
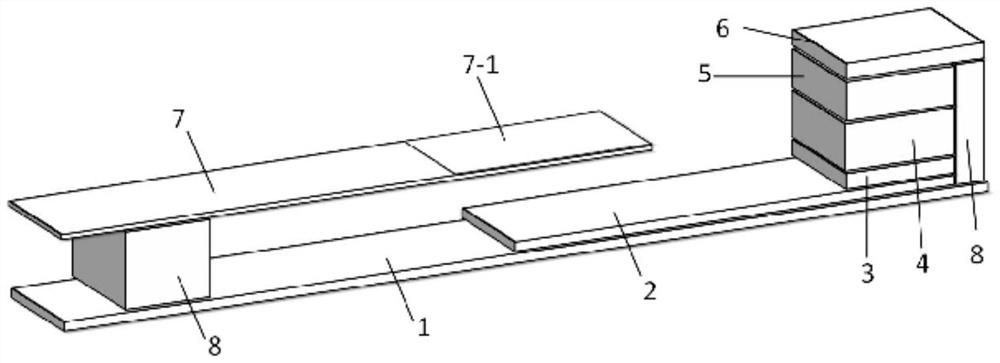
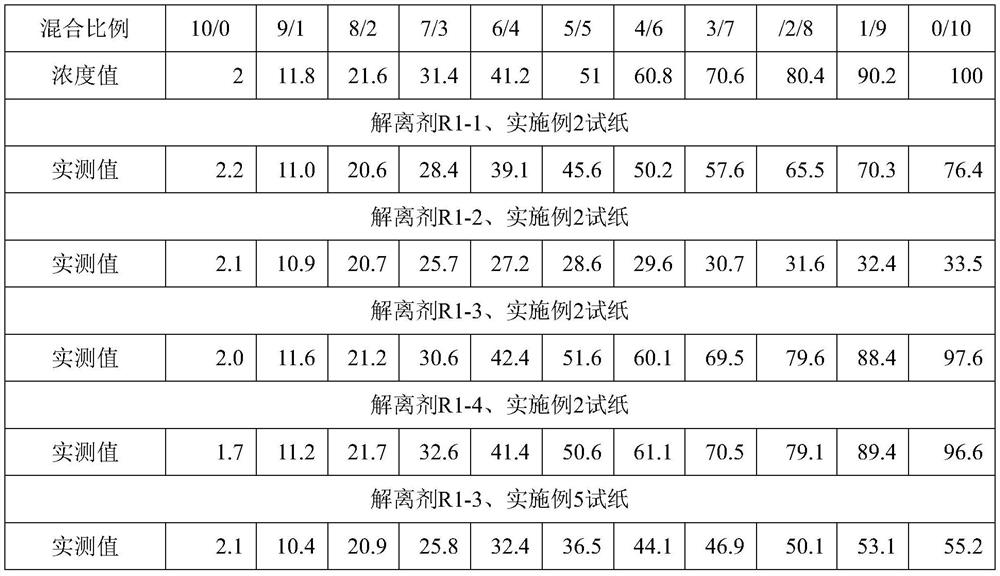
Dry homocysteine test card and application thereof
PendingCN111855648AMaterial analysis by observing effect on chemical indicatorHomocysteine testingBiology
The invention relates to a dry homocysteine test card and application thereof, the dry homocysteine test card comprises a dissociation liquid R1 and dry chemical test paper, the dissociation liquid R1comprises a reducing agent, serine and a buffer solution; the dry chemical test paper comprises a supporting layer, a chromatographic membrane, an enzyme pad, a blood filtering layer, an enzyme circulation reaction layer, a diffusion layer and a plastic sheet; the chromatographic membrane, the enzyme pad, the blood filtering layer, the enzyme circulation reaction layer and the diffusion layer aresequentially overlapped on the supporting layer from bottom to top, the length of the chromatographic membrane is greater than that of the enzyme pad, the blood filtering layer, the enzyme circulation reaction layer and the diffusion layer, and the chromatographic membrane is fixed at one end close to the supporting layer and extends towards the other end of the supporting layer; a plastic sheetis arranged near one end, far away from the enzyme pad, of the supporting layer, a color developing region is arranged on the plastic sheet, the plastic sheet is positioned above the chromatographic membrane and is parallel to the chromatographic membrane, and the color developing region covers partial region of the chromatographic membrane. The test card has the advantages of being capable of detecting all homocysteine in human blood and high in sensitivity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Liquid chromatography tandem mass spectrometry detection kit and detection method for testing folic acid metabolism derivatives of human body
The invention discloses a liquid chromatography tandem mass spectrometry detection kit and a detection method for testing folic acid metabolism derivatives of a human body, and belongs to the technical field of analysis and detection. The liquid chromatography tandem mass spectrometry detection kit for testing the folic acid metabolism derivatives of the human body can be used for detecting folic acid, tetrahydrofolic acid, 5-methyltetrahydrofolic acid, 5-formyltetrahydrofolic acid, 5,10-methylenetetrahydrofolate, S-adenosylmethionine, S-adenosylhomocysteine and homocysteine serum (plasma). The method has the characteristics of simplicity, rapidness, strong specificity, high sensitivity, comprehensive detection indexes and the like; the method can provide a reference basis for knowing the folic acid metabolism condition in clinical application.
Owner:广东南芯医疗科技有限公司 +1
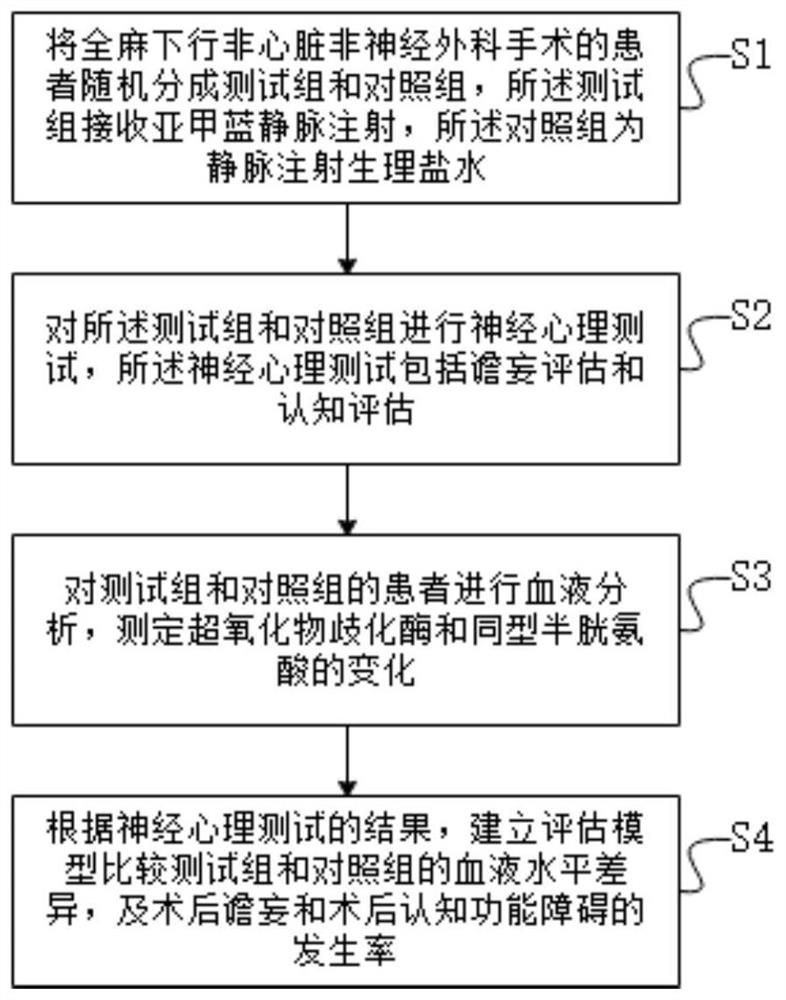
Method, system and device for prevention and assessment of postoperative cognitive impairment
PendingCN111956245AFunction increaseDiagnostic signal processingSensorsSurgical operationPostoperative cognitive dysfunction
The invention provides a method, system and device for prevention and assessment of postoperative cognitive impairment. Patients subjected to non-cardiac non-nerve surgical operations under general anesthesia are randomly divided into a testing group and a control group, wherein the testing group accepts methylene blue intravenous injection, and the control group is subject to intravenous injection of normal saline; neuropsychology tests are conducted on the testing group and the control group, and the neuropsychology tests include delirium assessment and cognition assessment; blood analysis is conducted on the patients in the testing group and the control group, and changes in superoxide dismutase and homocysteine are tested; and according to results of the neuropsychology tests, assessment models are established to compare differences of blood levels between the testing group and the control group as well as occurrence rates of postoperative delirium and postoperative cognitive impairment.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
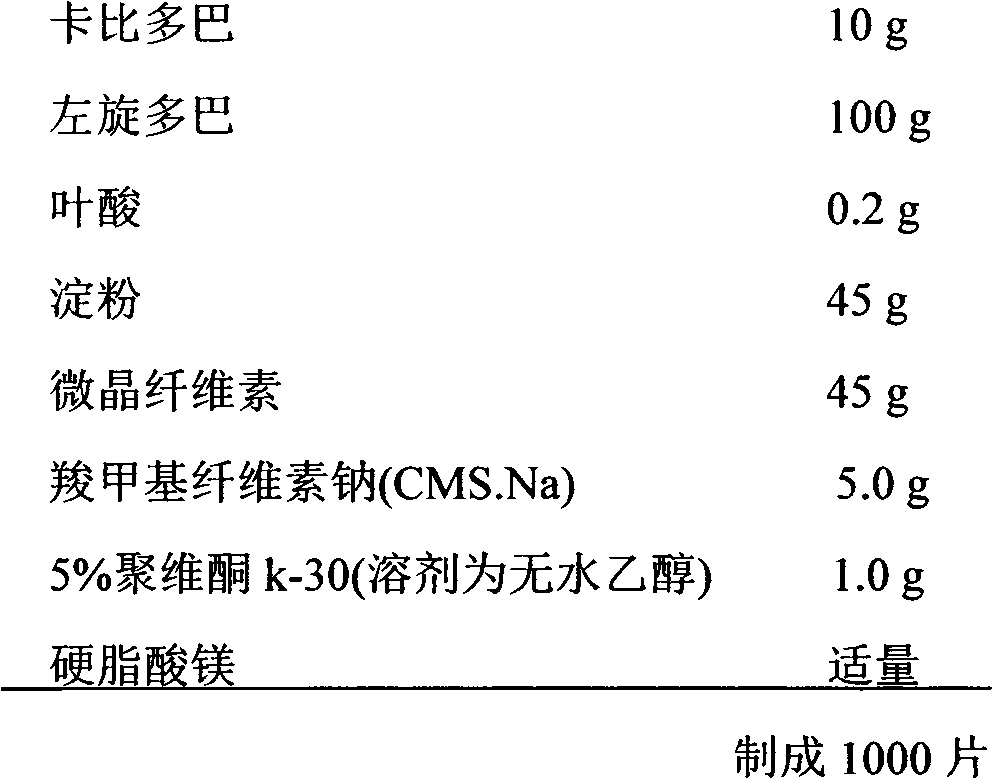
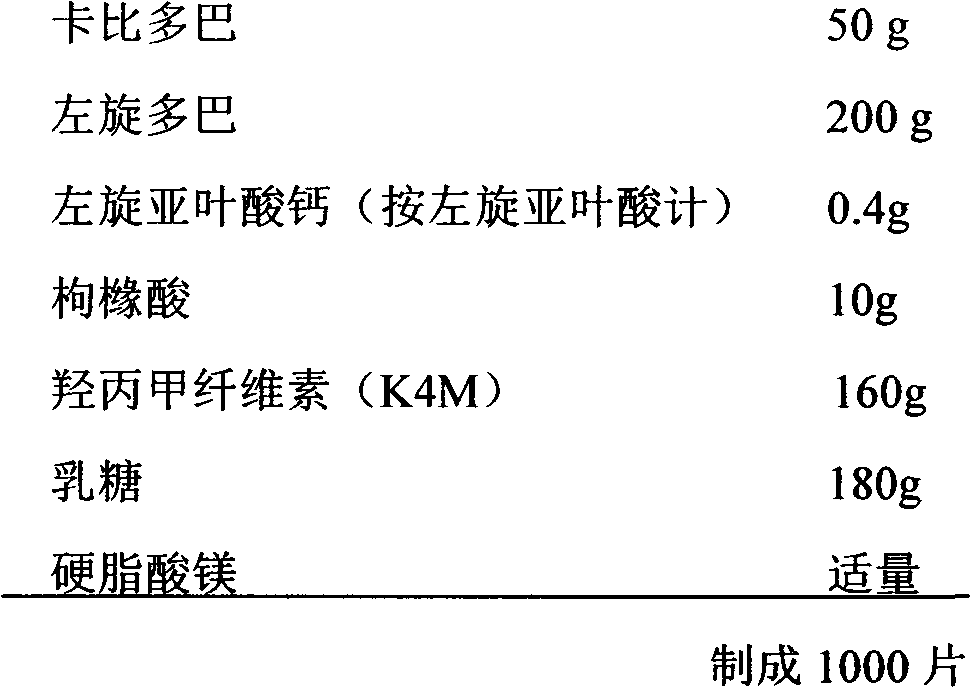
Carbidopa/levodopa/folic acid compound medicine composition and use thereof
InactiveCN103127116AGood curative effectPrevent and reduce the risk of strokeOrganic active ingredientsNervous disorderPharmaceutical medicineCarbidopa
The invention relates to a carbidopa / levodopa / folic acid compound medicine composition and the use thereof, and belongs to the technical field of pharmacy. The medicine combination comprises the carbidopa with an officinal dose, the levodopa with an officinal dose, the folic acid compounds with an officinal dose, and a carrier which can be accepted in pharmacy. The dose of the carbidopa is 10-60 mg, the dose of the levodopa is 100-300 mg, and the dose of the folic acid compounds is 0.2-1.6 mg. The medicine combination has the advantages that through synergistic effect of multiple target points, curative effect on the Parkinson's disease is improved, life quality of a patient is improved, through a homocysteine (Hcy) target point, cerebral apoplexy of the patient with the Parkinson's disease can be effectively prevented, occurring risk of the cerebral apoplexy can be effectively lowered, and in addition, the patient can conveniently take medicine.
Owner:SUZHOU FAMO BIOLOGICAL TECH
Reversible inhibitors of S-adenosyl-L-homocysteine hydrolase and uses thereof
The present invention provides compositions and methods for reversibly inhibiting S-adenosyl-L-homocysteine (SAH) hydrolase. The compounds of the present invention can be used as an anti-hemorrhagic viral infection agent, an immunosuppressant, a homocysteine lowering agent, or an anti-neoplasm agent. The compositions and methods of the present invention can be used for the prevention and treatment of hemorrhagic virus infection, autoimmune diseases, autograft rejection, neoplasm, hyperhomocysteineuria, cardiovascular disease, stroke, Alzheimer's disease, multiple sclerosis or diabetes. The compound of the present invention and / or used in the present invention has the formula (I):wherein Z is selected from the group consisting of carbon and nitrogen,R1 and R2 are the same or different, and are selected from the group consisting of Hydrogen, hydroxy, alkyl, cycloalkyl, alkenyl, alkoxy, amino, aryl, heteroaryl, and halogen;R3 and R4 are the same or different and are selected from the group consisting of Hydrogen, alkyl, acetyl, alkenyl, aryl, and heteroaryl;X is selected from the group consisting of oxygen, nitrogen, and sulfur; andY is selected from the group consisting of hydrogen, a C1-10 alkyl group, alkenyl, vinyl, aryl, and heteroaryl, provided that the compound is not (4-adenine-9-yl)-2-hydroxybutanoic acid.
Owner:NINGBO ZIYUAN PHARMA INC
Kit for determining homocysteine and preparation method thereof
InactiveCN106053830AImprove enzyme stabilityEasy to operateBiological testingS-Adenosyl-l-methioninePhosphate
The invention discloses a kit for determining homocysteine and a preparation method thereof. The kit is composed of dual-liquid components of a reagent R1and a reagent R2 which are independent from each other. The reagent R1 is prepared from a phosphate buffer, sodium chloride, S-adenosylmethionine, a reduced coenzyme II, tri(2-carboxyl) phosphine hydrogen chloride and sodium azide; the reagent R2 is prepared from phosphate buffer, HCY transmethylase, glutamate dehydrogenase, S-adenosylhomocysteine hydrolase, bovine serum albumin and sodium azide. The preparation method comprises the steps that the reagents are prepared according to the component content; a to-be-tested sample is mixed with the reagent R1 and the reagent R2 to perform a sufficient reaction; a fully automatic biochemical analyzer is used for determining the reacted absorptivity difference; according to the absorptivity change value, the concentration of homocysteine in the sample is worked out. The kit has the advantages of being convenient to operate, high in accuracy and the like.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Methods and compositions for assaying homocysteine
ActiveUS8476034B2Microbiological testing/measurementBiological material analysisCo substrateHomocysteine
This invention relates generally to the field of homocysteine detection. In particular, the invention provides a method for determining homocysteine presence or concentration in samples, which method comprises: contacting a sample containing or suspected of containing Hcy with a Hcy co-substrate and a Hcy converting enzyme in a Hcy conversion reaction to form a Hcy conversion product and a Hcy co-substrate conversion product; and assessing the Hcy co-substrate conversion product to determine the presence, absence and / or amount of the Hcy in the sample. The Hcy co-substrate conversion product may be assessed directly, or it may be assessed by further conversion of the Hcy co-substrate conversion product into another material by the action of one or more additional enzymes. A kit for assaying homocysteine based on the same principle is also provided.
Owner:DIAZYME LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com