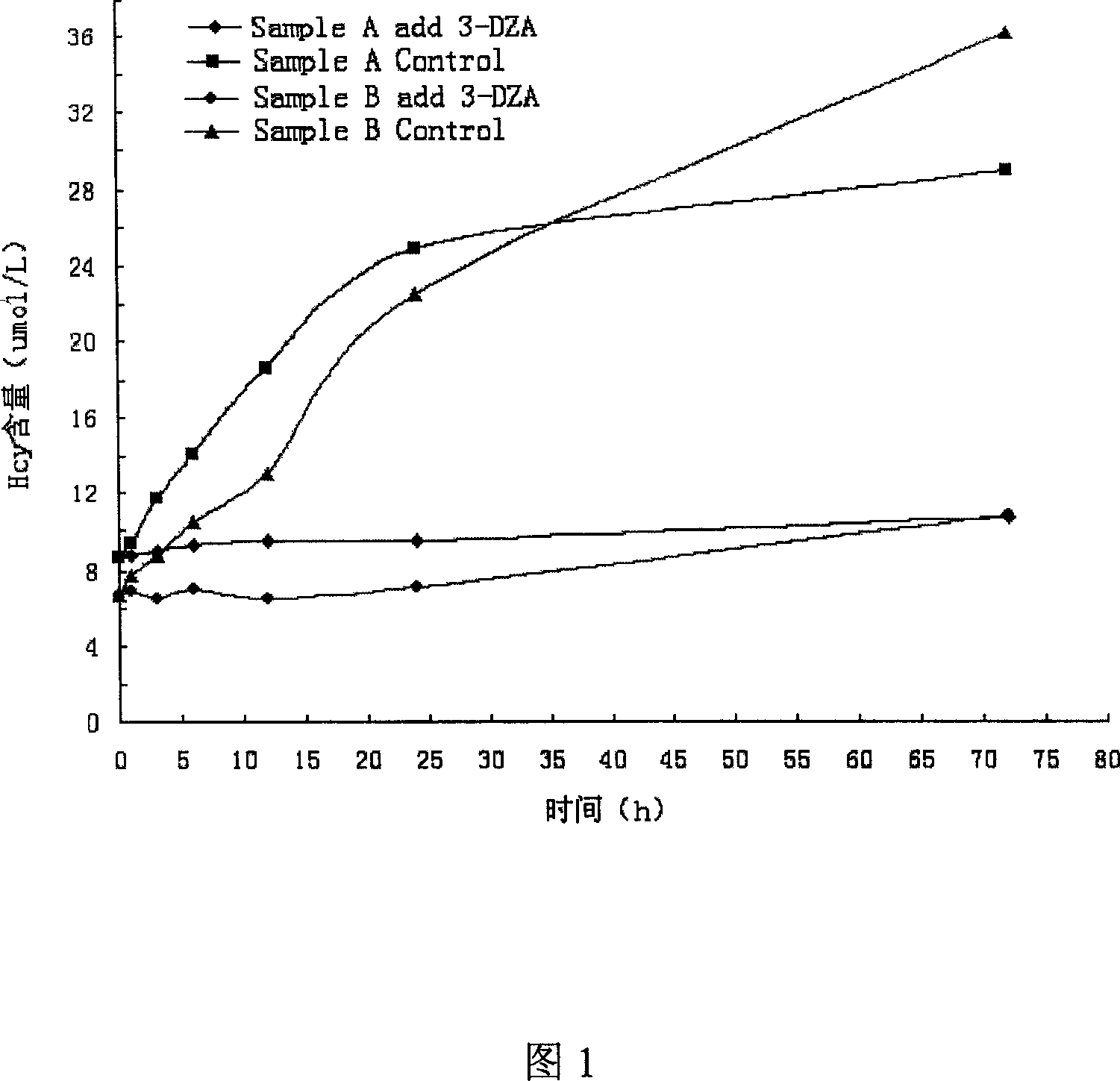
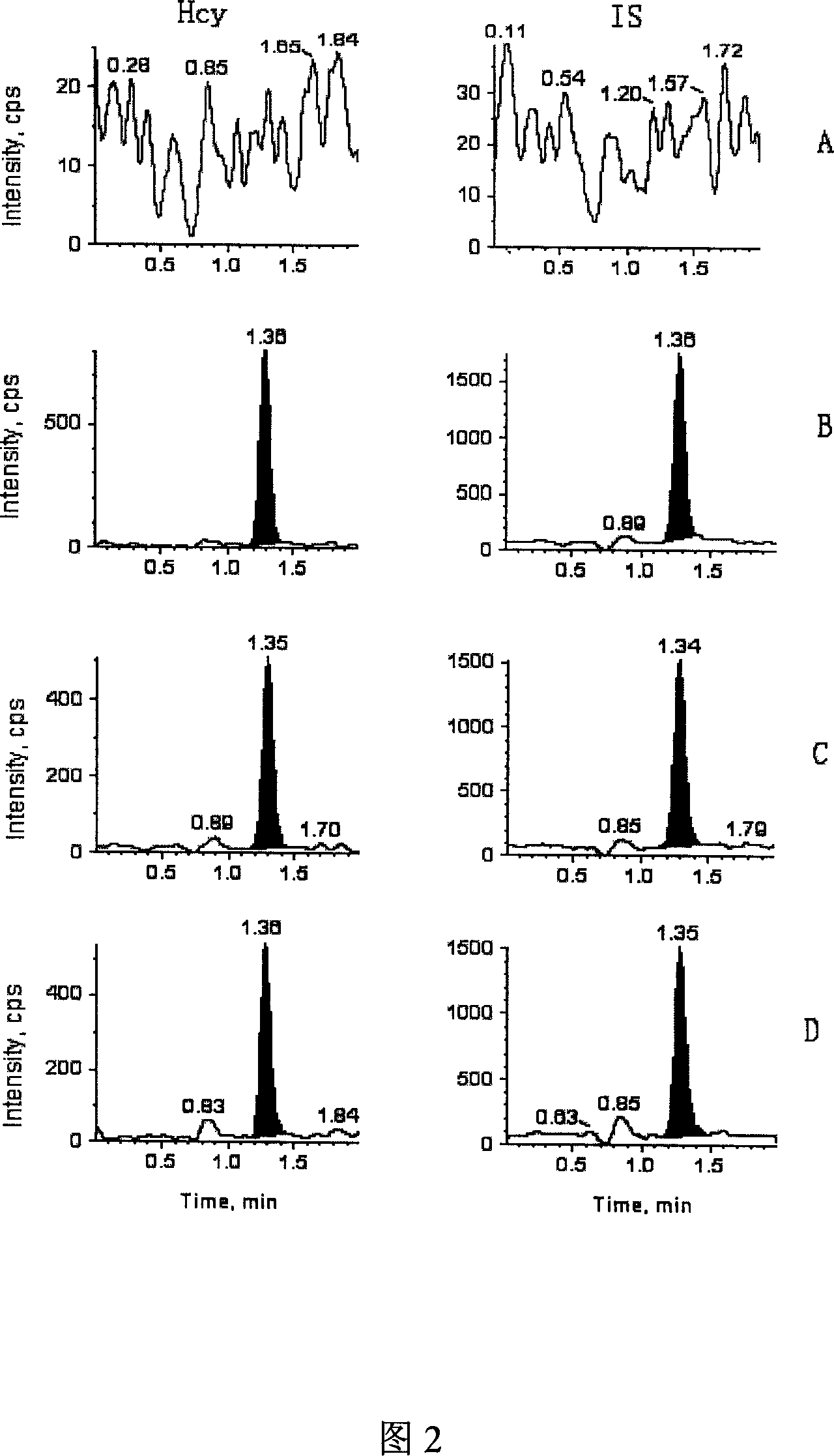
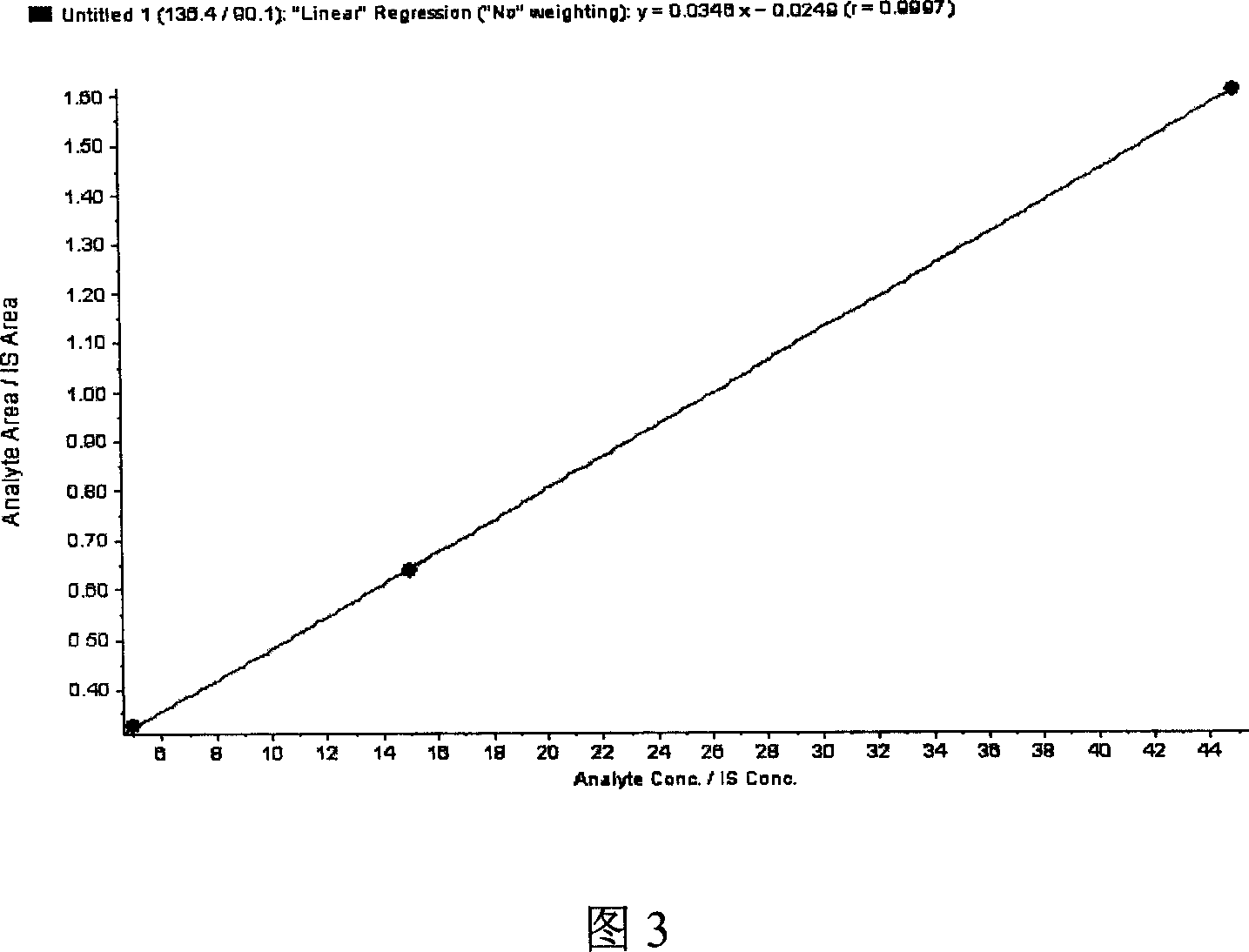High-sensitive blood-plasma total homocysteine detection reagent box
A homocysteine and kit technology, which is applied in the field of high-sensitivity plasma total homocysteine determination kits, can solve the problems of poor specificity, difficulty in meeting high-level clinical research, high technical requirements, and high reagent prices. Achieve the effect of optimized mobile phase, accurate detection results and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Kit reagent preparation:
[0035] Sample tube: 2 μg of 3-deazaadenosine was added as a stabilizer;
[0036] Reference substance: dialyzed blank serum with a Hcy concentration of 0, adding Hcy chemicals to prepare Hcy concentrations of 45, 15 and 5 μmol / L;
[0037] Quality control: dialyzed blank serum with a Hcy concentration of 0, adding Hcy chemicals to prepare Hcy concentrations of 30 μmol / L and 10 μmol / L;
[0038] Internal standard solution: DL-homocystine-D8 aqueous solution, the concentration is 10 μmol / L;
[0039] Reducing agent: 1,4-dithiothreitol aqueous solution, the concentration is 75mmol / L;
[0040] Protein precipitant: trichloroacetic acid aqueous solution, the concentration is 15% (w / v)
[0041] 2. Blood sample collection: Collect venous blood in a sample tube, centrifuge the plasma (4000rpm×10min) within 8 hours, and store it at room temperature.
[0042]3. Sample processing: Take 50 μl of the plasma to be tested and place it in a centrifuge tube,...
Embodiment 2
[0056] The kit, blood sample collection and pretreatment methods are the same as in Example 1 except that the reducing agent 1,4-dithiothreitol aqueous solution has a concentration of 100 mmol / L, and the mass spectrometry conditions are also the same as in Example 1.
[0057] Chromatographic conditions: using Aquasil octadecylsilane bonded silica gel as the stationary phase, mobile phase A: 0.1% formic acid in methanol solution, mobile phase B: 0.1% formic acid in water, the ratio is 10:90; isocratic elution, flow rate 0.25mL / min, inject 5μl.
[0058] Under these conditions, the retention time of Hcy and the internal standard are both 1.05 min, the total analysis time of a sample is 2.0 min, and the minimum detection limit is 0.1 μmol / L (signal-to-noise ratio ≥ 5).
[0059] The total homocysteine concentration in the sample was calculated by the peak area or peak height according to the internal standard method.
Embodiment 3
[0061] 2 μg of sodium heparin was added to the sample tube as a stabilizer, and the other kits, blood sample collection and pretreatment methods were the same as in Example 1, and the chromatographic and mass spectrometry conditions were also the same as in Example 1.
[0062] Calculate the total homocysteine concentration in the sample by the peak area or peak height method according to the internal standard method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com