Synthesis of multi-signal fluorescent probe and application of multi-signal fluorescent probe in detection of Cys, GSH and Hcy
A technology of fluorescent probes and fluorescent molecular probes, applied in the field of analytical chemistry, can solve problems such as low sensitivity, poor selectivity, and unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
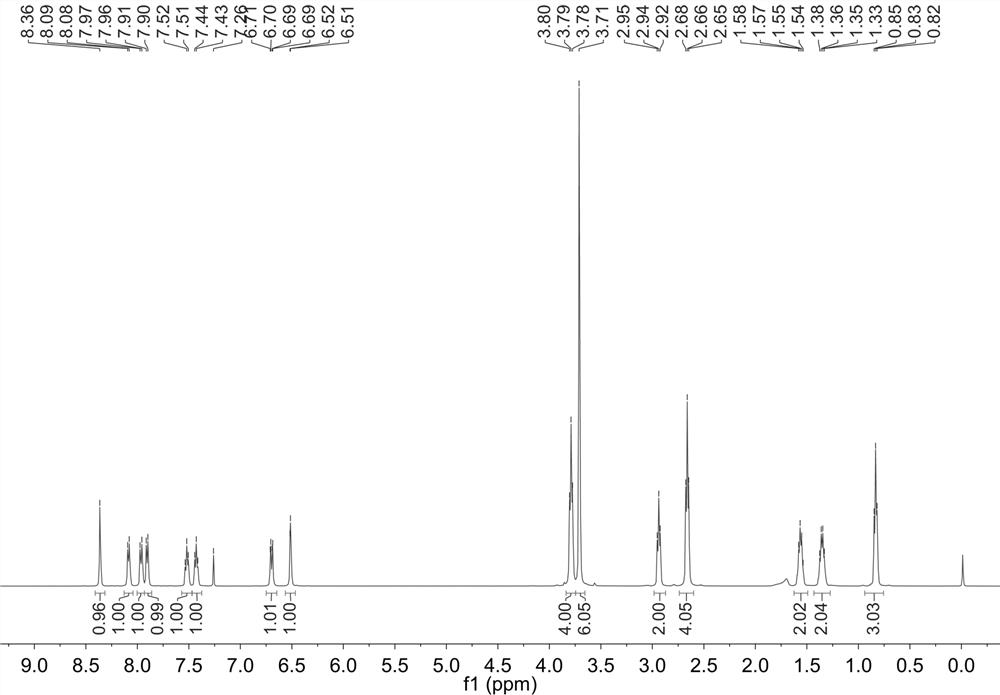
[0023] Example 1. Synthesis of 3,3'-((4-(butylthio)-3-formyl-2-oxo-2 H -pyran-7-yl)azadiyl)dimethyl dipropionate
[0024] a. Add 3.0 g (5.68 mmol) 3,3'-((4-chloro-3-formyl-2-oxo-2 H -pyran-7-yl)azadiyl)dimethyl dipropionate was added to 20 mL of dichloromethane, and then 615.2 mg (6.82 mmol) of n-butanethiol and 690.3 mg (5.68 mmol) of triethylamine were added at room temperature Stirring and reacting for 6 hours;
[0025] b. After the reaction was completed, the solvent was spin-dried on a rotary evaporator, and purified by column chromatography to obtain 2.0 g of a yellow solid product with a yield of 78.3%.
Embodiment 2
[0026] Example 2. Synthetic probe dimethyl 3,3'-((3-(2-(benzo[ d ]thiazol-2-yl)-2-cyanoethenyl)-4-(butylthio)-2-oxo-2 H -pyran-7-yl)azadiyl)( E )-dipropionate
[0027] . 1.0 g (2.22 mmol) of 3,3'-((4-(butylthio)-3-formyl-2-oxo-2 H -pyran-7-yl)azadiyl)dimethyl dipropionate, 391.2 mg (2.25 mmol) 2-(benzo[ d ]thiazol-2-yl) acetonitrile and 38.3 mg (0.22 mmol) p-toluenesulfonic acid were added to 20 mL of absolute ethanol, and stirred at room temperature for 12 hours;
[0028] . After the reaction is complete, filter and recrystallize the filter cake with absolute ethanol to obtain the multi-signal fluorescent probe of the present invention.
Embodiment 3
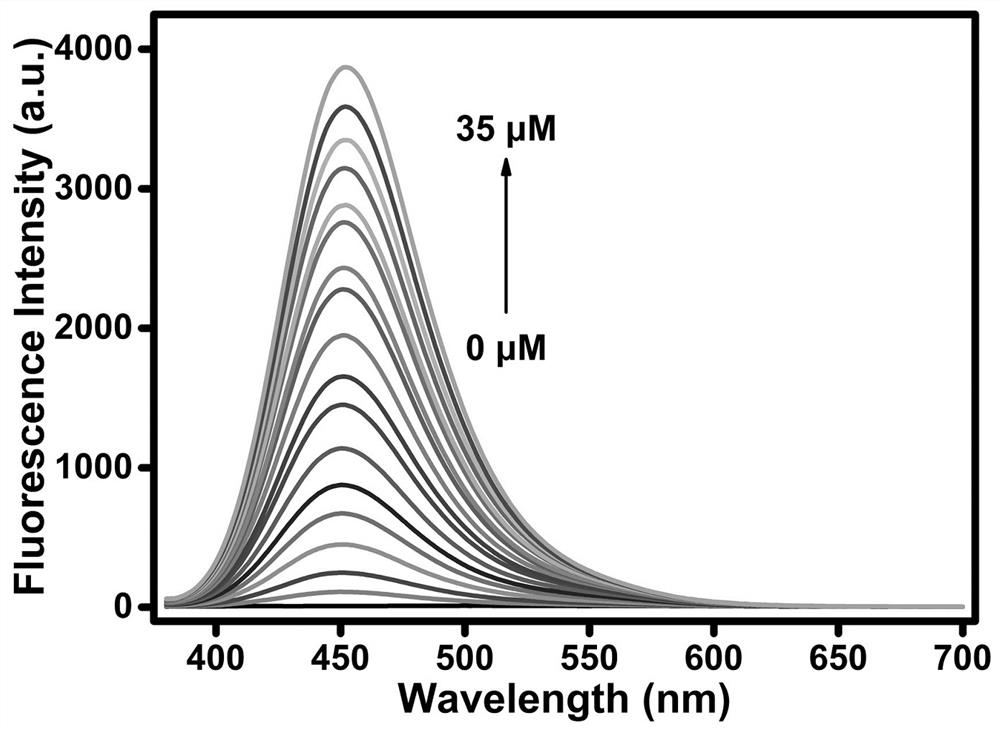
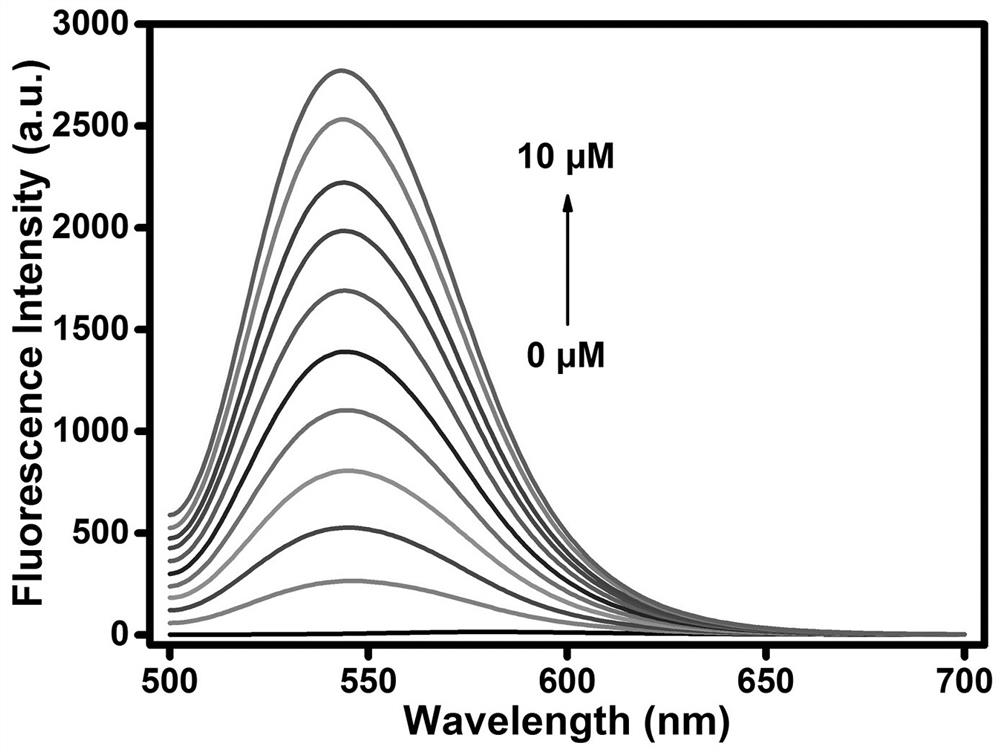
[0029] Example 3. The application of the multi-signal fluorescent probe of the present invention to simultaneously distinguish Cys, GSH and Hcy
[0030]The spectroscopic property experiment of detecting Cys, GSH and Hcy with fluorescent molecular probes according to the present invention: the probes were dissolved in dimethyl sulfoxide (DMSO) to prepare a probe solution with a concentration of 1 mM, and the concentration of each preparation was 1 mM. Cys, GSH and Hcy in mM in water. The specific test method for detecting Cys / GSH is as follows: Take 20 μL of probe solution (1 mM), 780 μL of analytically pure DMSO, the required amount of 1 mM Cys / GSH aqueous solution and the required amount of PBS buffered aqueous solution in 2 In the sample tube of mL, the final volume ratio of the organic phase and the aqueous phase was maintained at 4:6 (the total volume of each test sample was 2 mL) for all tests. After shaking for 30 minutes at room temperature, the 360 and 415 nm The ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com