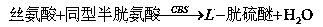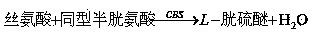Hcy detecting method and detecting kit
A homocysteine and determination method technology, applied in the field of homocysteine determination methods and determination kits, can solve time-consuming problems and achieve the effects of long storage time, good stability and low test cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 (double dose)
[0087] Prepare the homocysteine assay kit according to the following ingredients and dosage:
[0088] Reagent 1:
[0089] Lactate dehydrogenase (LD) 35KU / L;
[0090] Serine (SER) 0.76 mmol / L;
[0091] NADH 0.47 mmol / l;
[0092] Reagent 2:
[0093] Cystathionine β-synthase (CBS) 20KU / L;
[0094] Cystathionine β-decomposing enzyme (CBL) 10KU / L.
[0095] The reagents 1 and 2 may also include a buffer composed of 12mmol / L pH8.3 Tris-HCL and KCL60mmol / L; a mixture of glycerol and mannitol, wherein glycerol:mannitol=15:1. The buffer and stabilizer can significantly improve the stability of the kit.
[0096] Detection method:
[0097] 1. Basic parameters
[0098] Method: rate method Sample / reagent: 1 / 17
[0099] Dominant wavelength: 340nm Reaction temperature: 37°C
[0100] Secondary wavelength: 405nm Response time: 10min
[0101] 2. Determination
[0102] Blank Sample Calibration
[0103] Normal s...
Embodiment 2
[0118] Example 2 (single dose)
[0119] Prepare the homocysteine assay kit according to the following ingredients and dosage:
[0120] Lactate dehydrogenase (LD) 30.33KU / L;
[0121] Serine (SER) 0.66 mmol / L;
[0122] NADH 0.41 mmol / l;
[0123] Cystathionine β-synthase (CBS) 2.67KU / L;
[0124] Cystathionine β-decomposing enzyme (CBL) 1.33KU / L.
[0125] The reagent may also include a buffer composed of 12mmol / L pH8.3 Tris-HCL and KCL60mmol / L; a mixture of glycerol and mannitol, wherein glycerol:mannitol=15:1.
[0126] The detection method can be adjusted accordingly with reference to Example 1. for example:
[0127] Detection method:
[0128] 1. Basic parameters
[0129] Method: rate method Sample / reagent: 1 / 17
[0130] Dominant wavelength: 340nm Reaction temperature: 37°C
[0131]Secondary wavelength: 405nm Response time: 10min
[0132] 2. Determination
[0133] Blank Sample Calibration
[0134] Normal saline / H2O (μl) 16 0 0
...
Embodiment 3
[0147] Example 3 (three doses)
[0148] Prepare the homocysteine assay kit according to the following ingredients and dosage:
[0149] Reagent 1: NADH 0.94mmol / l;
[0150] Reagent 2:
[0151] Lactate dehydrogenase (LD) 70KU / L;
[0152] Serine (SER) 1.52mmol / L;
[0153] Reagent 3:
[0154] Cystathionine β-synthase (CBS) 20KU / L;
[0155] Cystathionine β-decomposing enzyme (CBL) 10KU / L.
[0156] The reagents 1, 2 and 3 may also include a buffer composed of 12mmol / L pH8.3 Tris-HCL and KCL60mmol / L; a stabilizer sodium azide.
[0157] The detection method can be adjusted accordingly with reference to Example 1. for example:
[0158] Detection method:
[0159] 1. Basic parameters
[0160] Method: rate method Sample / reagent: 1 / 17
[0161] Dominant wavelength: 340nm Reaction temperature: 37°C
[0162] Secondary wavelength: 405nm Response time: 10min
[0163] 2. Determination
[0164] Blank Sample Calibration
[0165] Normal saline / H2O (μl) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com