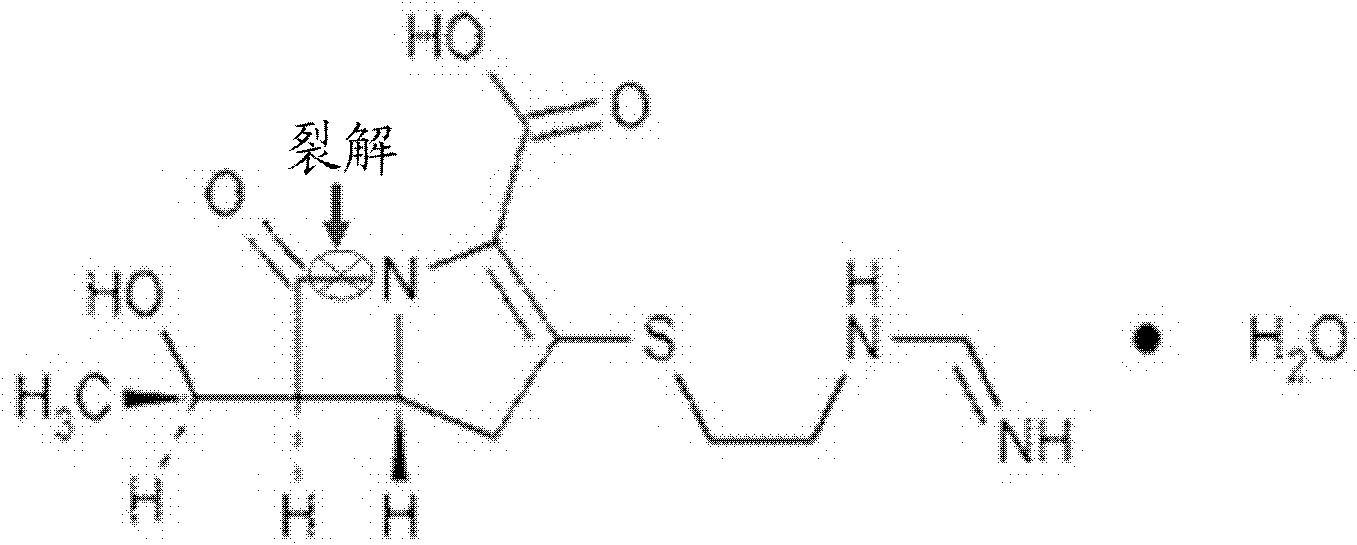N,N'-asymmetric diayl substitution urea compound and preparation method and purpose thereof
A compound, asymmetric technology, applied in the N field, can solve the problems of generating by-products and decreasing yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
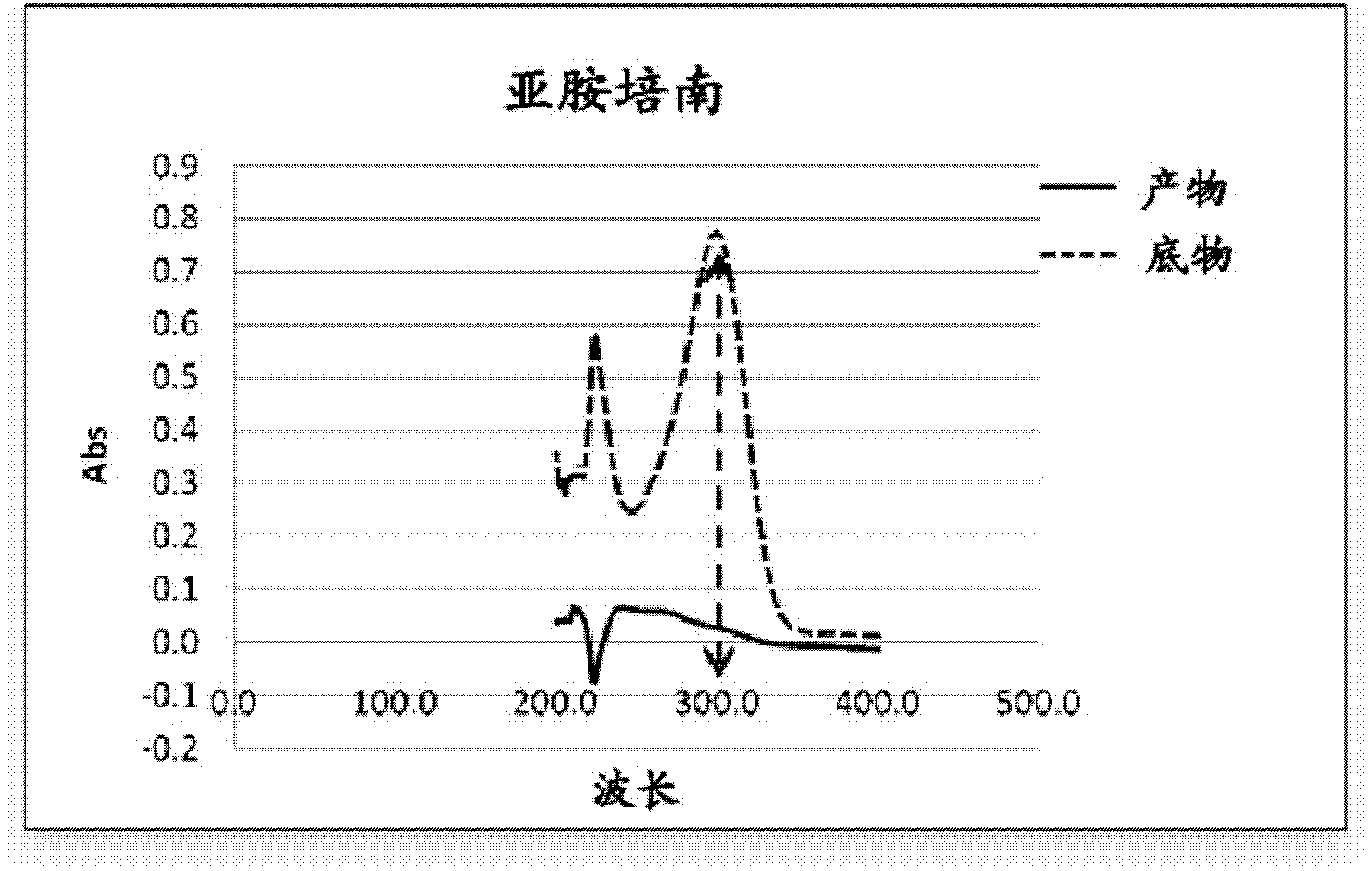
[0070] Step 1. Preparation of NDM-1 substrate stock solution
[0071] Imipenem monohydrate (Imipenem monohydrate, purchased from Sigma Company) was dissolved in 50 mM HEPES (purchased from BioBasic Company) to prepare a 10 mM substrate stock solution for future use.
[0072] Step 2. Treatment of Compounds
[0073] Compound in 95%DMSO+5%ddH 2 O, and prepared to a solution with a concentration of 100mM, and then placed the prepared compound solution in a 1.5ml ep tube, and stored it at 4°C for use.
[0074] Step 3. Preparation of NDM-1 protein buffer
[0075] NDM-1 (provided by the MDC protein purification group of our laboratory, the preparation method refers to Yu Guo, Jing Wang et al., A structural view of the antibiotic degradation enzyme NDM-1 from a superbug. Protein & Cell, 2011, 2(5): 384 -394) was dissolved in protein buffer (pH=6.8), and was prepared into 50nM NDM-1 protein buffer, wherein the protein buffer contained 50mM HEPES, 5μM ZnCl 2 (purchased from BioBasic...
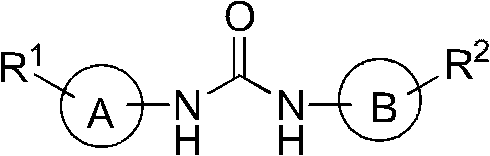
Embodiment 1
[0088] The preparation of embodiment 11-phenyl-3-naphthyl urea
[0089]
[0090] Triphosgene (10 mmol) dissolved in 20 ml dichloromethane was added dropwise to 1-aminonaphthalene (10 mmol) dissolved in 20 ml dichloromethane, then triethylamine (3 ml) dissolved in 10 ml dichloromethane was added dropwise , stirred at room temperature for 30 minutes; the solution was transferred to a rotary evaporator, and evaporated to dryness, the residue was dissolved in 20ml of dichloromethane, and aniline (10mmol) dissolved in 20ml of dichloromethane was added, and the mixture was refluxed for 30min; the solution was transferred to a rotary evaporator In the evaporator, the obtained residue was dissolved with 30ml of acetone, and 30ml of water was added, and the precipitate was filtered with suction and washed with water-acetone (1:1, 4×5ml) to obtain the product. The yield was 88%. Gray powder, M.P. 223-224°C. 1 H NMR (400MHz, DMSO-d6, δin ppm): 9.07(s, 1H), 8.78(s, 1H), 8.14(d, J=8.4...
Embodiment 2
[0091] Preparation of Example 21-(4-tert-butylphenyl)-3-(1-naphthyl)urea
[0092]
[0093] The aniline in Example 1 is replaced with p-tert-butylaniline, and all the other steps are the same as in Example 1. The yield was 88%. Gray powder, M.P. 239-241°C. 1 H NMR (400MHz, DMSO-d6, δinppm): 8.99(s, 1H), 8.74(s, 1H), 8.14(d, J=8.4Hz, 1H), 8.04(d, J=8.4Hz, 1H), 7.95(d, J=7.8Hz, 1H), 7.60(m, 3H), 7.50(t, J=7.8Hz, 1H), 7.43(d, J=9Hz, 2H), 7.33(d, J=9Hz, 2H), 1.29(s, 9H); ESI-MS m / z: 319.20 ([M+H + ]).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com