Oxidized iso-aporphine alkaloid derivative, synthetic method and application
A technology of alkaloid derivatives and oxidized isoaporphine, applied in the field of medicine, can solve the problems that have not been seen before, and achieve the effects of good medicinal value and significant in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
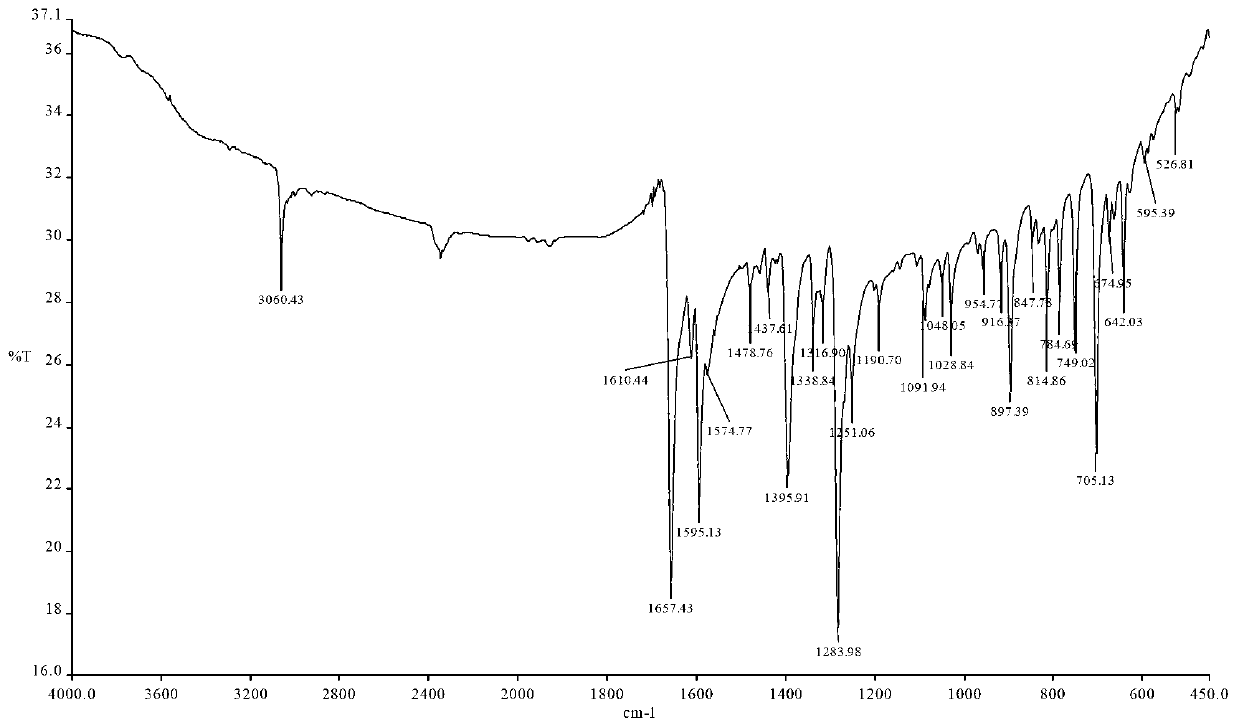
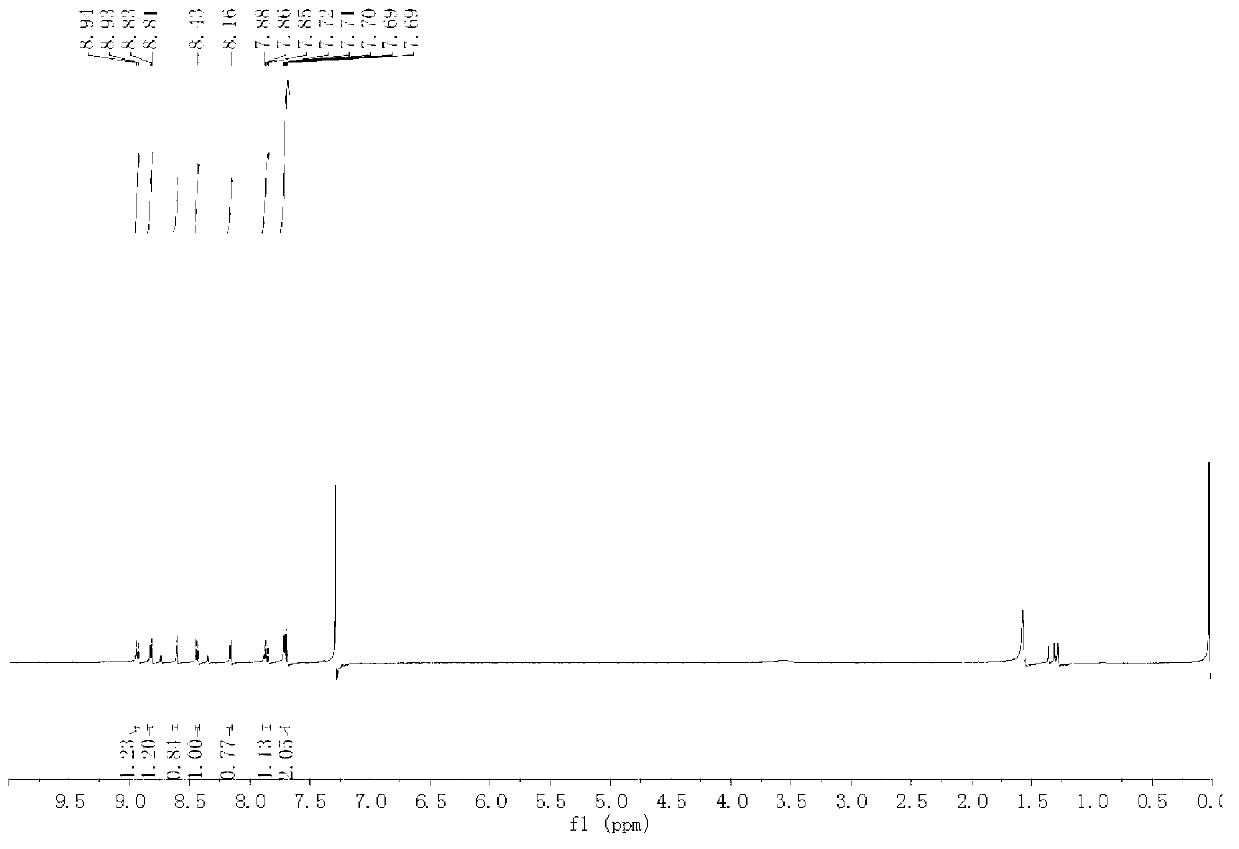
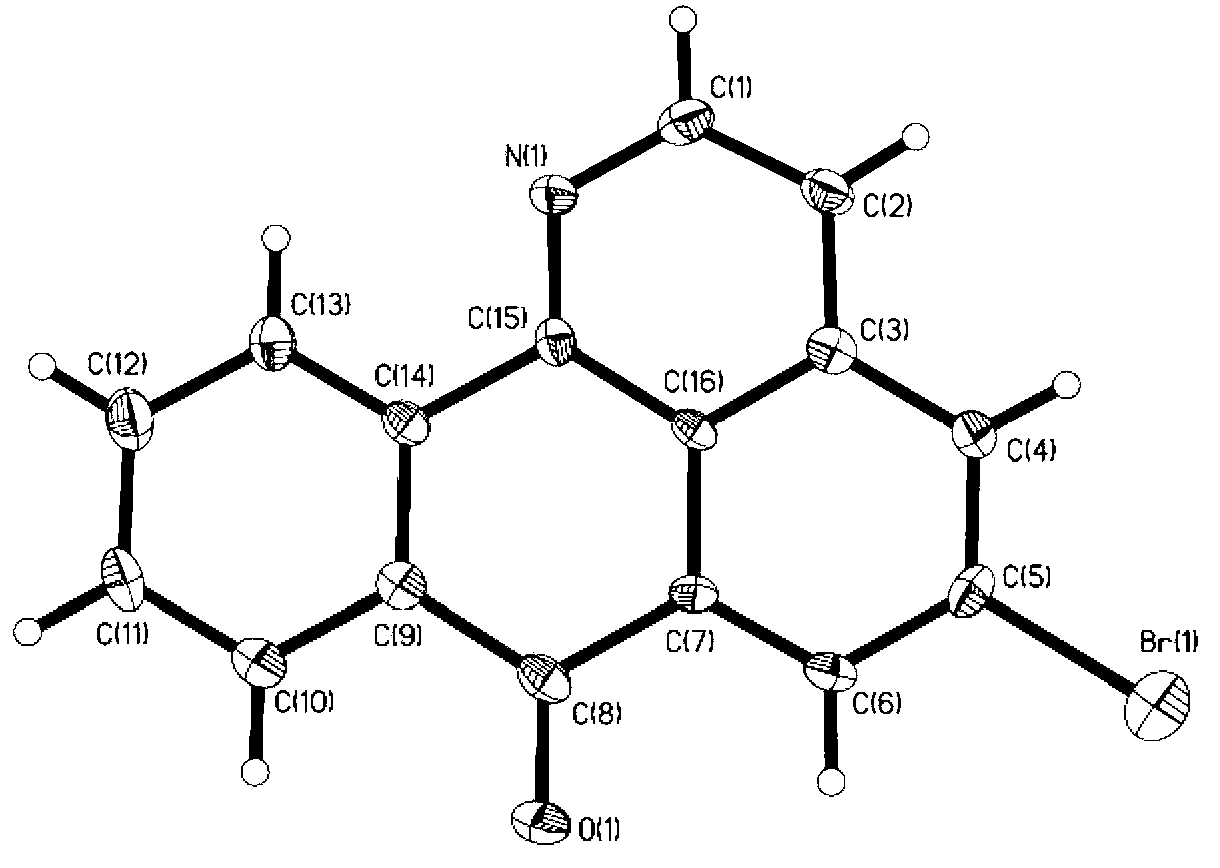
[0043] Embodiment 1: Synthesis of 1-nitrogen-5-bromoxabenzanthrone
[0044] 1) Synthesis of intermediate 1 (4-bromophenethylamine):
[0045] Dissolve 0.2mol of lithium aluminum hydride in 200mL of anhydrous ether, and dissolve 0.2mol of 4-bromophenylacetonitrile in 50mL of anhydrous ether. Slowly add the anhydrous ether solution of 4-bromophenylacetonitrile dropwise. After the addition is complete, stir and react at room temperature for 6 hours, neutralize with saturated sodium sulfate solution until no bubbles are generated, filter the reaction solution with suction, wash the filter residue with chloroform, and depressurize The solvent was distilled off to obtain intermediate 1 (colorless and slightly viscous 4-bromophenethylamine liquid), with a yield of 40%;
[0046] 2) Synthesis of Intermediate 2:
[0047] Mix and dissolve 0.1 mol of phthalic anhydride and 0.1 mol of intermediate 1 in 200 mL of ethanol, reflux at 90°C for 8 hours, cool the reaction solution to room tempe...
Embodiment 2
[0059] Get 0.01mol of 1-nitrogen-5-bromoxabenzanthrone (prepared according to the method described in Example 1) and dissolve it in 25mL of n-pentanol, add 0.025mol of 2-(aminomethyl)pyridine, at 180°C Reflux reaction under the conditions until complete (TLC tracking detection, about 72h), cooling, a large amount of reddish-brown solids precipitated during the reaction, suction filtration, the filter residue was washed with ethanol and ether successively, and dried to obtain the crude product of the target product; the crude product of the target product was obtained with Chloroform (according to the amount of 60 milliliters of chloroform per gram of the target product crude product to calculate the amount of chloroform) after dissolving, go to silica gel column chromatography, elute with a mixed solvent composed of chloroform and methanol with a volume ratio of 50:1, and the eluent is evaporated to dryness solvent to obtain a reddish-brown solid product with a yield of about 9...
Embodiment 3
[0071] Take 0.01mol of 1-nitrogen-5-bromoxabenzanthrone and dissolve it in 50mL of n-pentanol, add 0.04mol of 2-(aminomethyl)pyridine, and reflux the reaction at 170°C until complete (TLC tracking detection , about 48h), cooling, the reaction process has a large amount of reddish-brown solid to separate out, suction filtration, filter residue is washed with ether, ethanol successively, dry, obtains target product crude product; The amount of chloroform is used to calculate the amount of chloroform) after dissolving, go to silica gel column chromatography, elute with a mixed solvent composed of chloroform and methanol with a volume ratio of 60:1, and the eluent is evaporated to dryness to obtain reddish-brown oxidized isoaporphine The yield of alkaloid derivative PPCA is about 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com