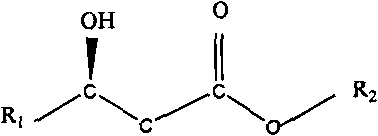
Biosynthesis preparation method of (R)-3-hydroxyalkanoate
A hydroxyalkanoate and biosynthesis technology, applied in fermentation and other directions, can solve problems such as poor stability, affecting the yield and purity of chiral alcohol, and the existence of monomer forms, so as to reduce dosage, reduce metabolic burden, and increase curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The present invention is further described below in conjunction with specific embodiment, but protection scope of the present invention is not limited thereto:
[0019] Enzyme extraction method was used to obtain β-ketothiolase (phbA), acetoacetyl-CoA reductase (phbB) and PHA synthase (phbC) necessary for the biosynthesis of poly(R)-3-hydroxyalkanoic acid; Technology Construct the above enzymes into the E. Coli gene to obtain a stable strain suitable for industrial production;
[0020] Bacterial growth medium: yeast powder 3g / L, peptone 8g / L, sodium chloride 10g / L, PH7.0
[0021] The obtained gene recombinant bacteria are fermented and cultured for 72 hours, centrifuged for solid-liquid separation, the organic phase is extracted from the solid phase, concentrated, and dried to obtain poly(R)-3-hydroxybutyric acid.
[0022] Fermentation medium: Na 2 HPO 4 .12H 2 O 3g / L, KH 2 PO 4 0.5g / L, (NH 4 ) 2 SO 4 1.0g / L, MgSO 4 0.5g / L NaCl 0.5g, glucose 60g / L;
[0023...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com