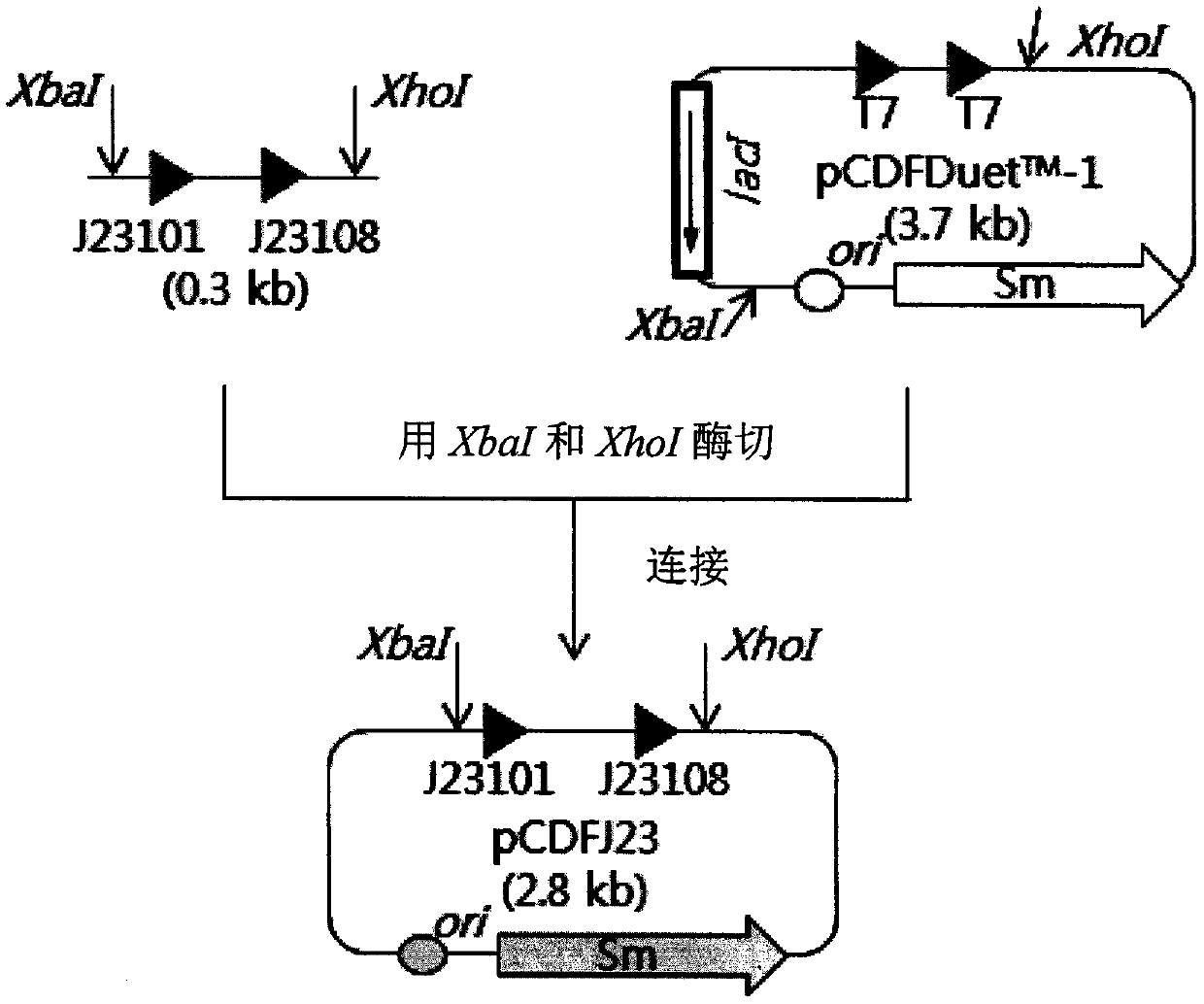
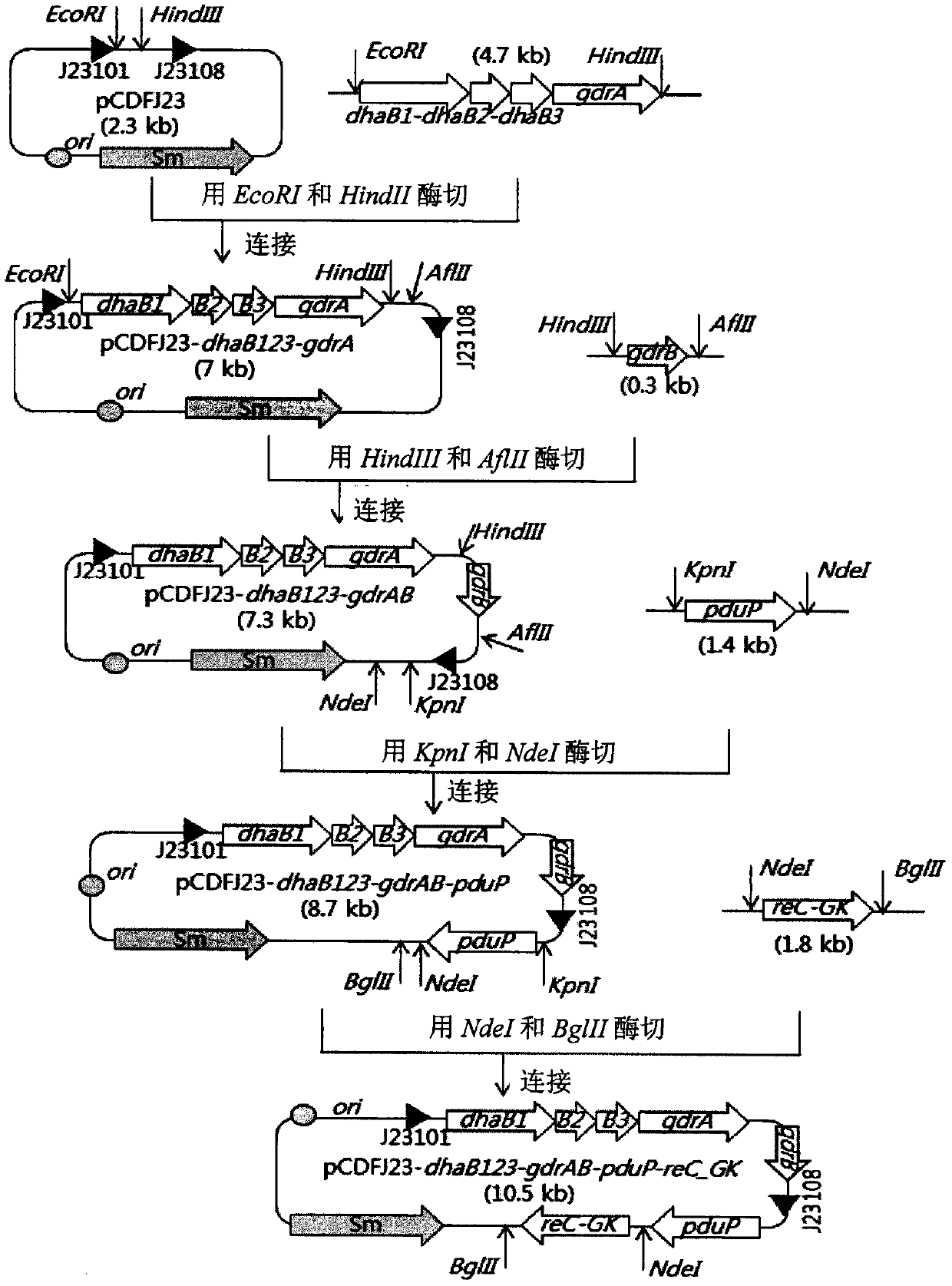
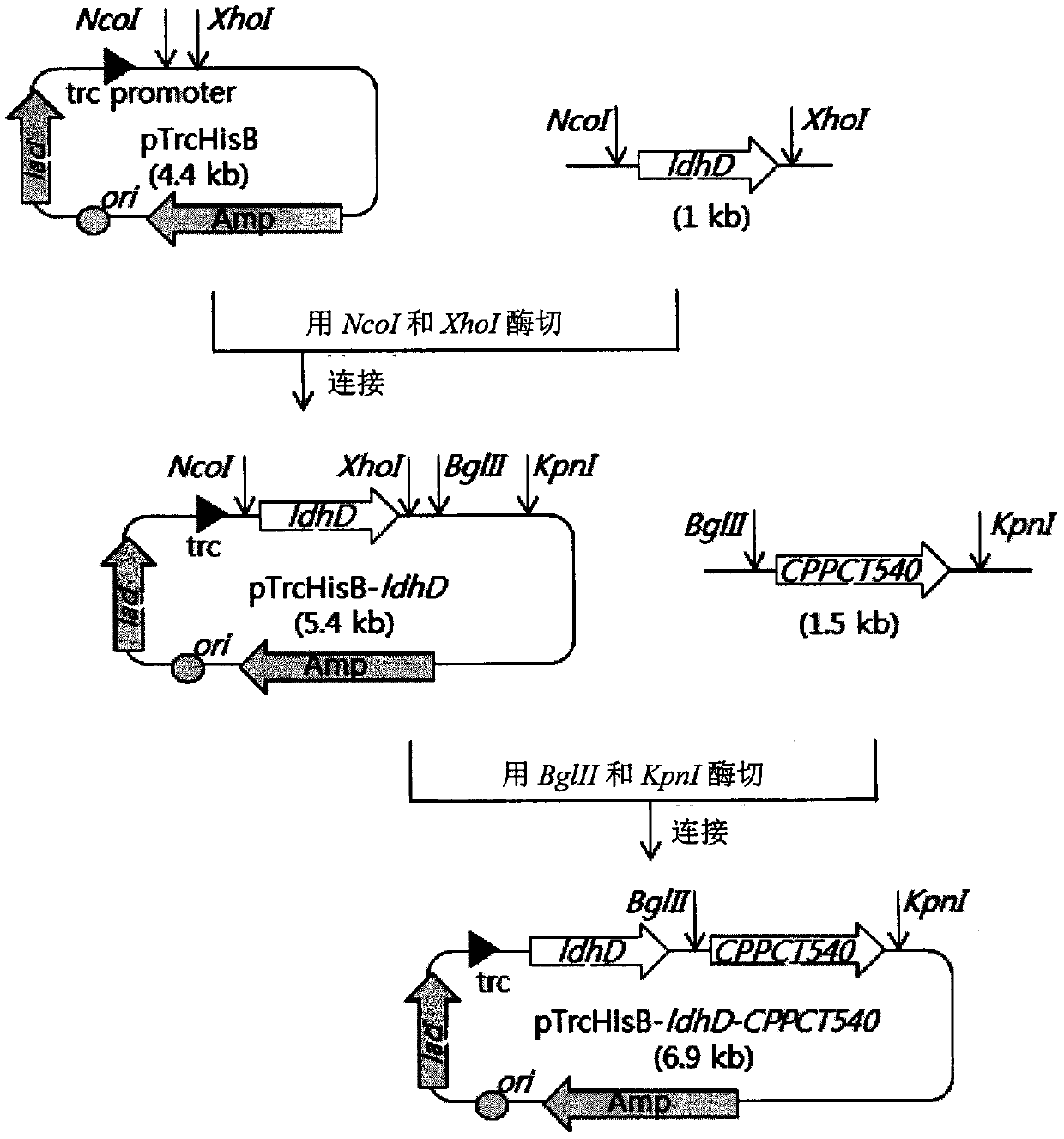
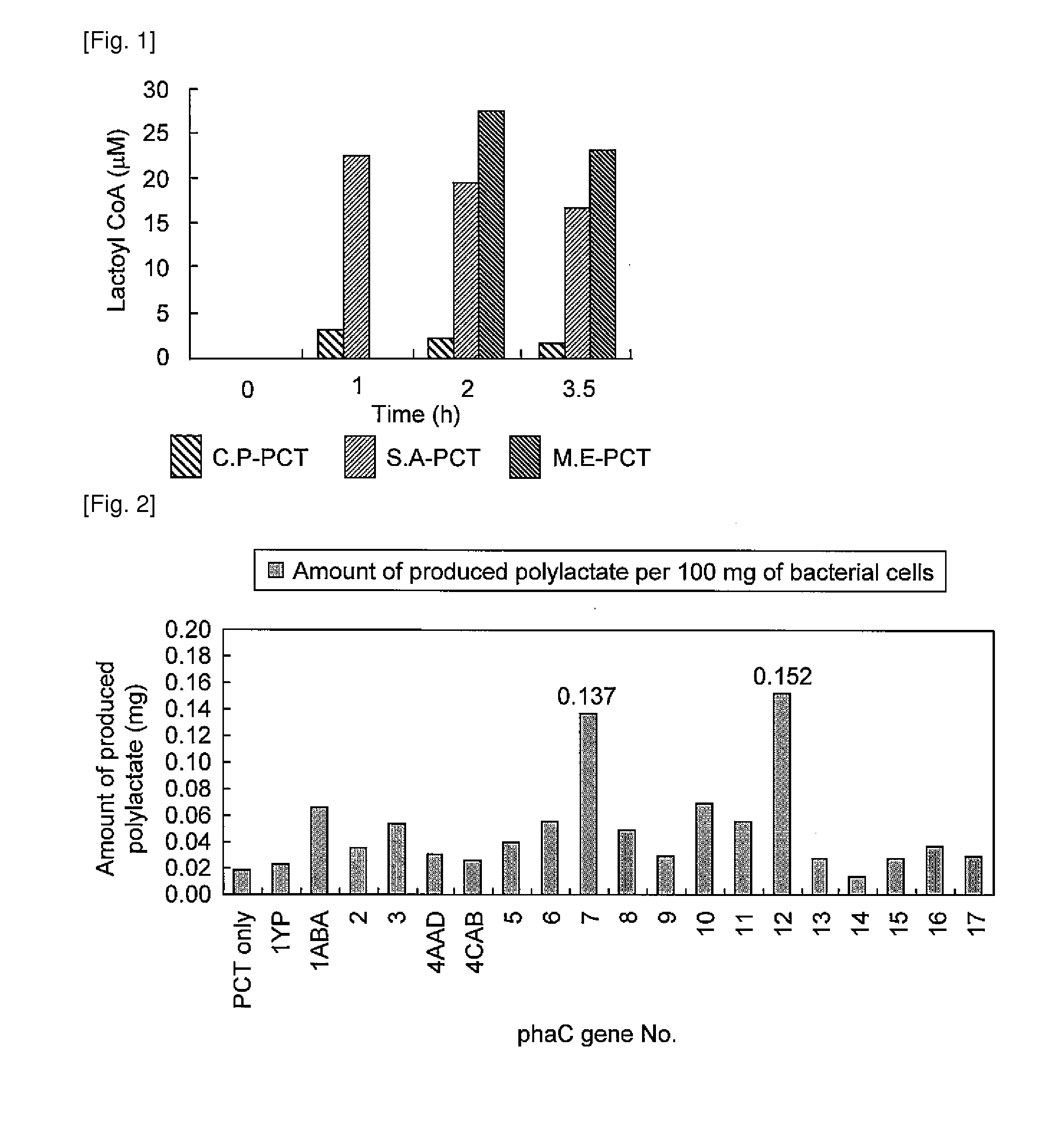
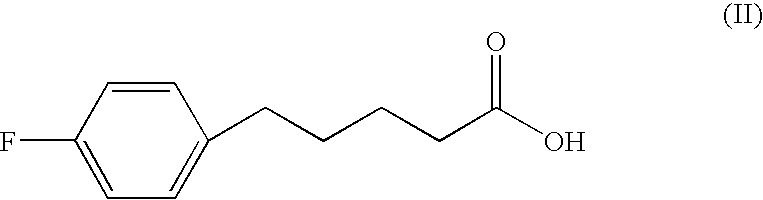
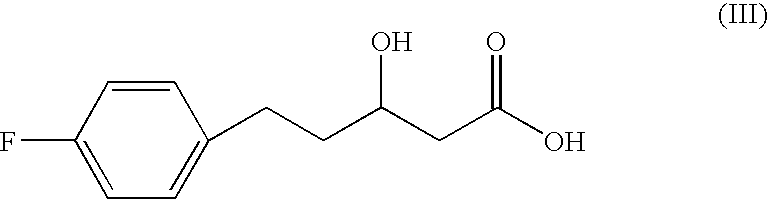
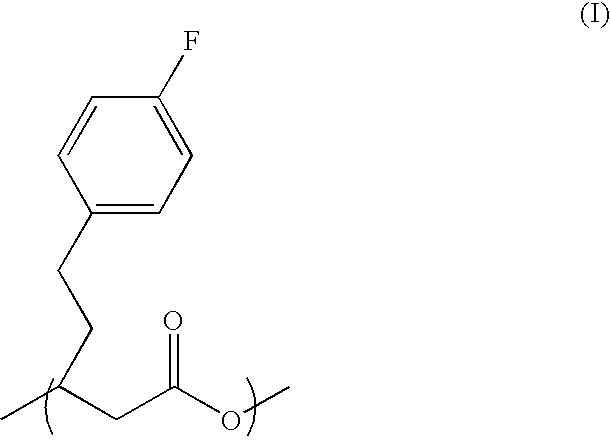
Patents
Literature
46 results about "PHA synthase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
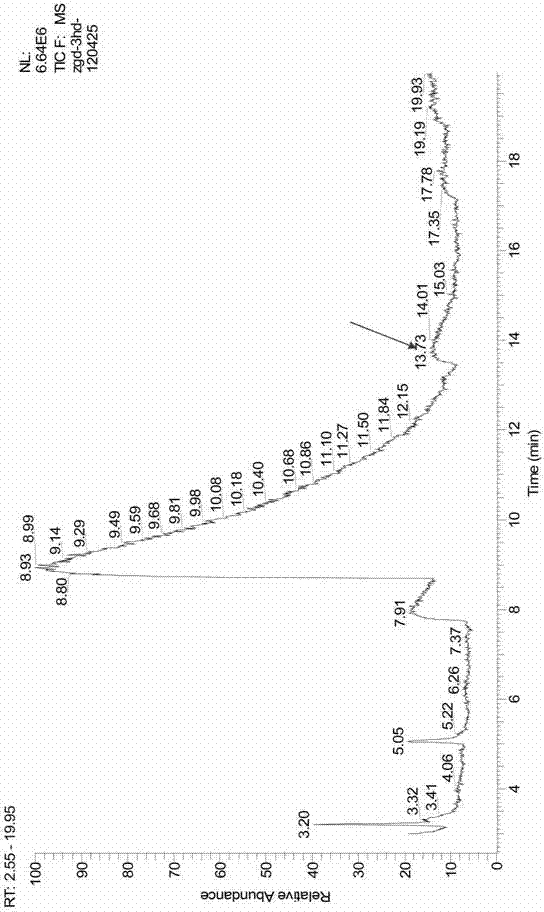
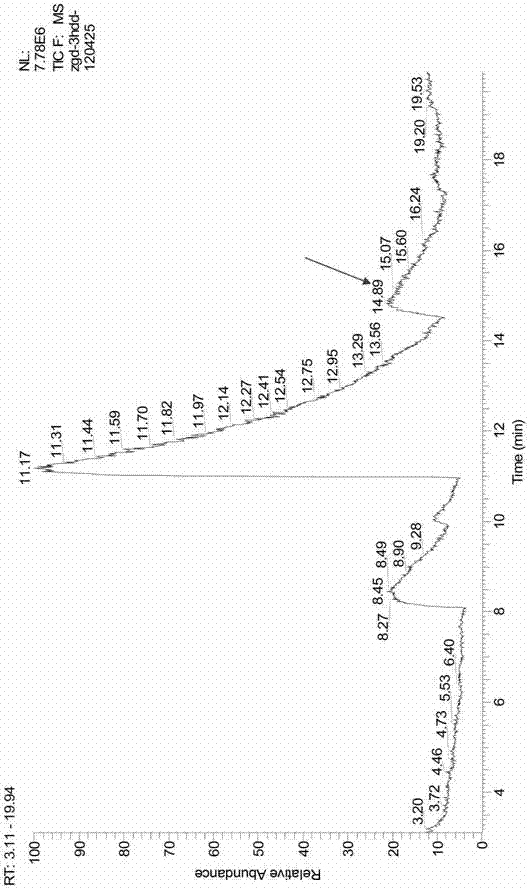
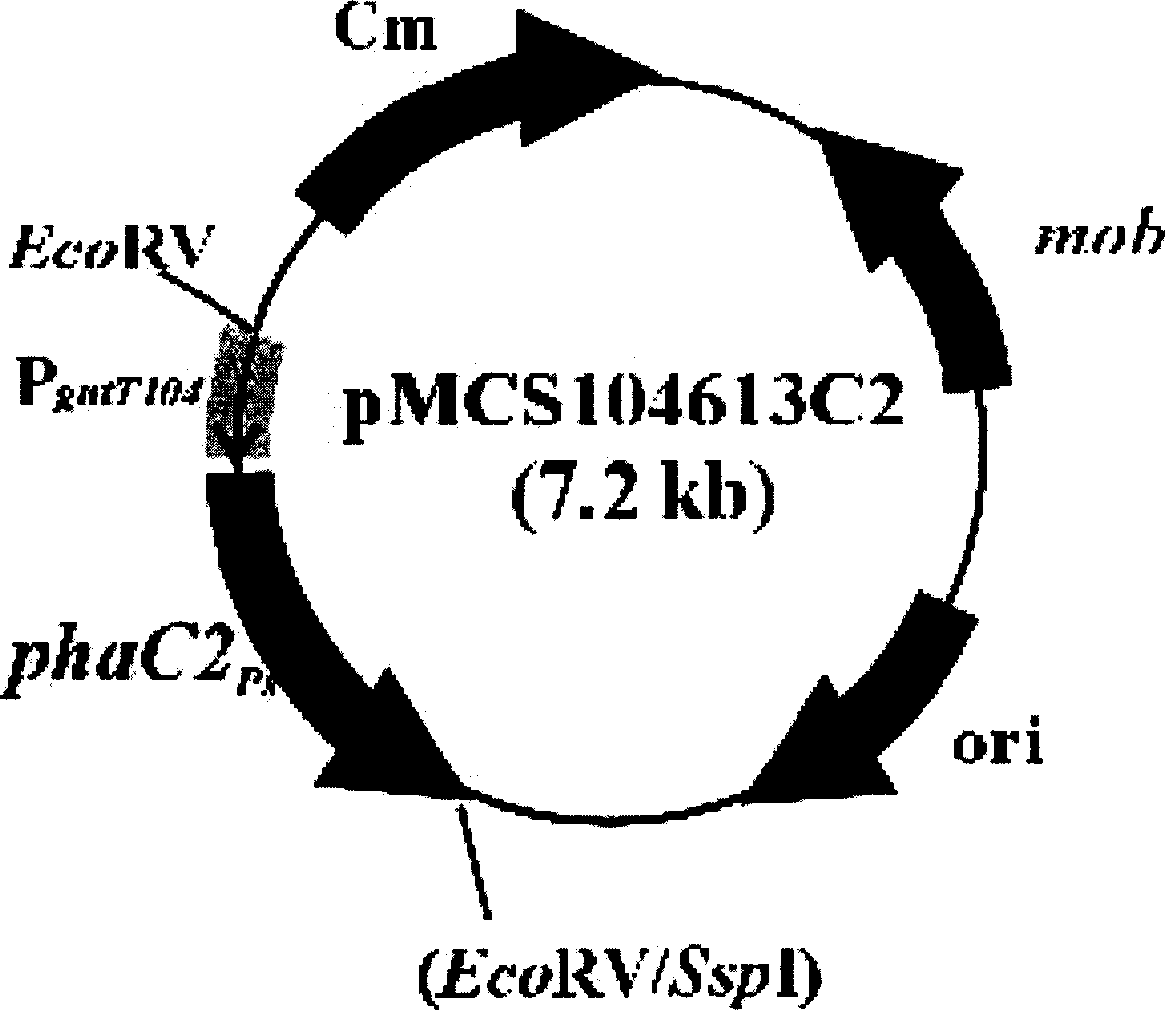
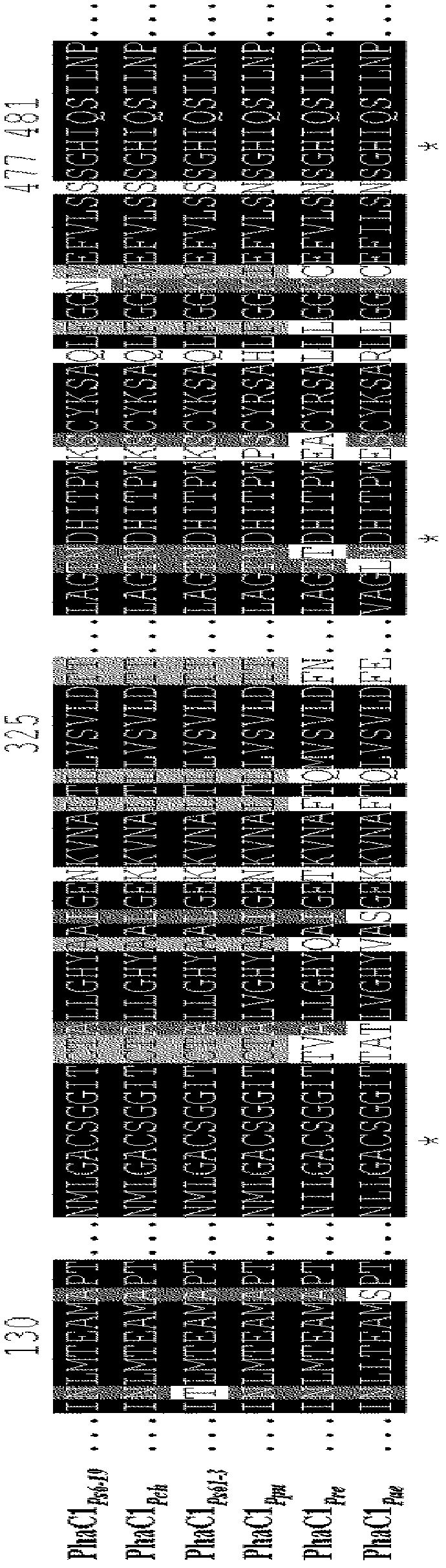
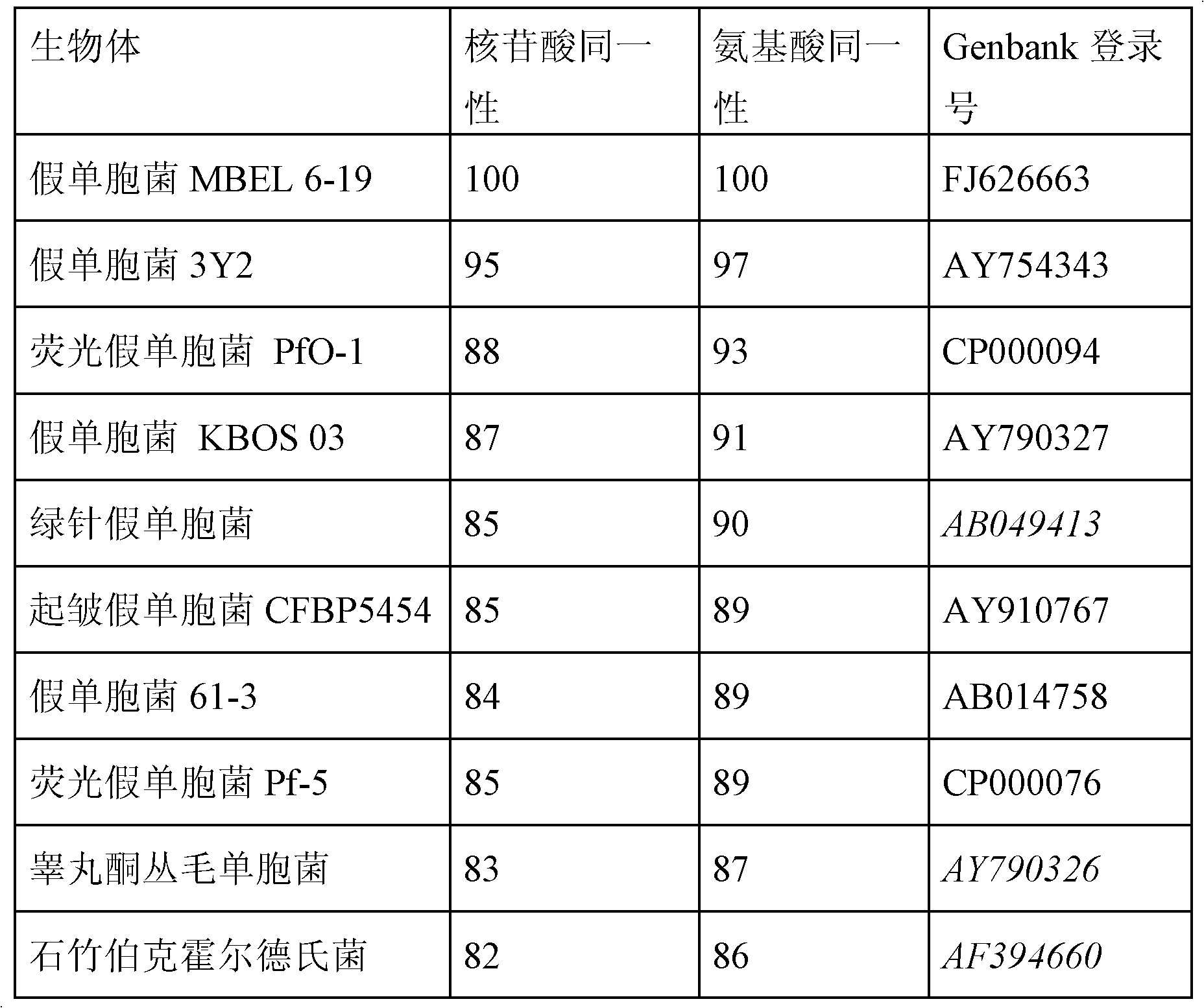
The PHA synthase from clone 18 showed the highest similarity (99%) with the PHA synthase 1 (PhaC 1) from Pseudomonas mendocina NK-01. The PHA MCL produced by NK-01 mainly consists of 3-hydroxyoctanoate (3HO) and 3-hydroxydecanoate (3HD) monomers (13).
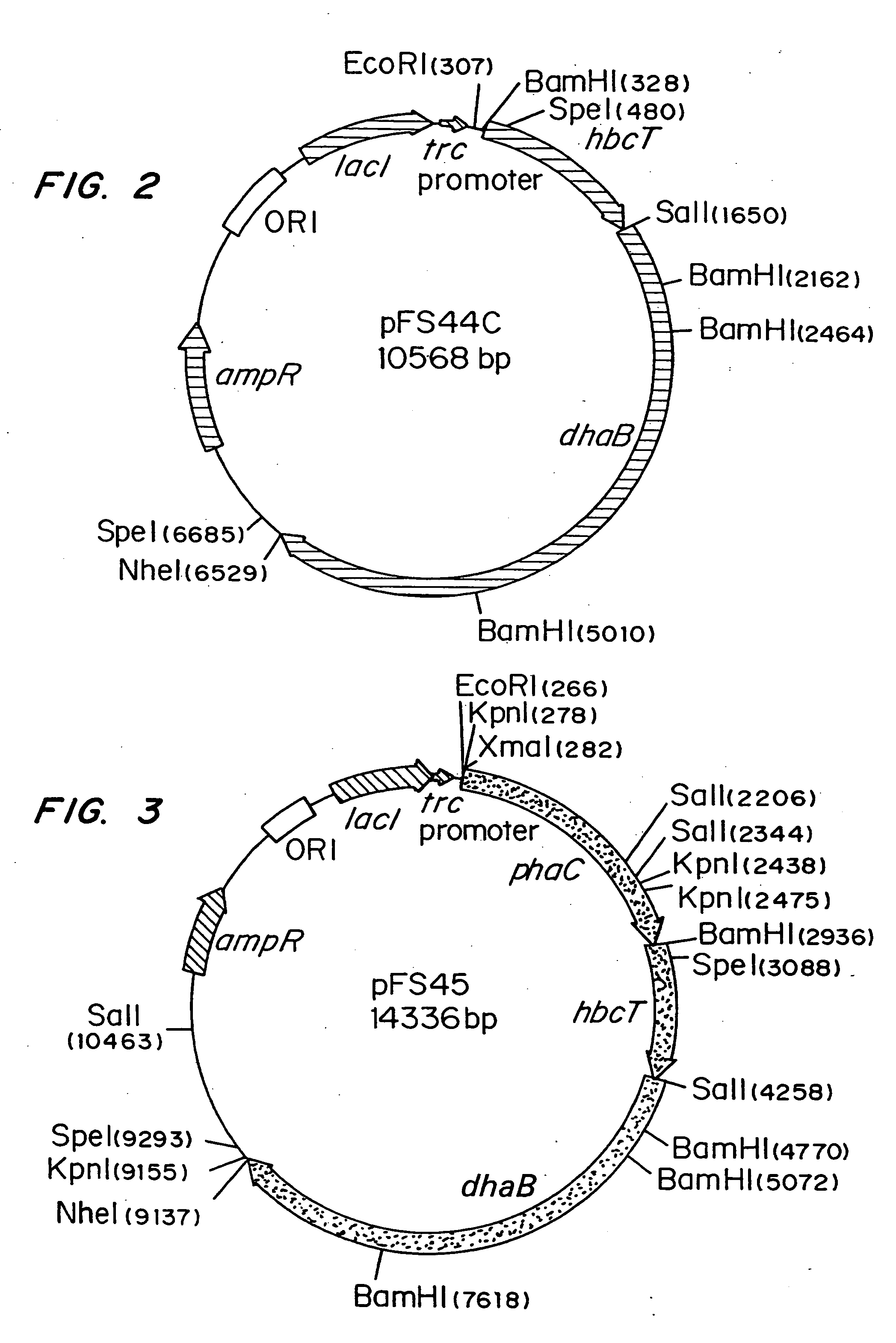
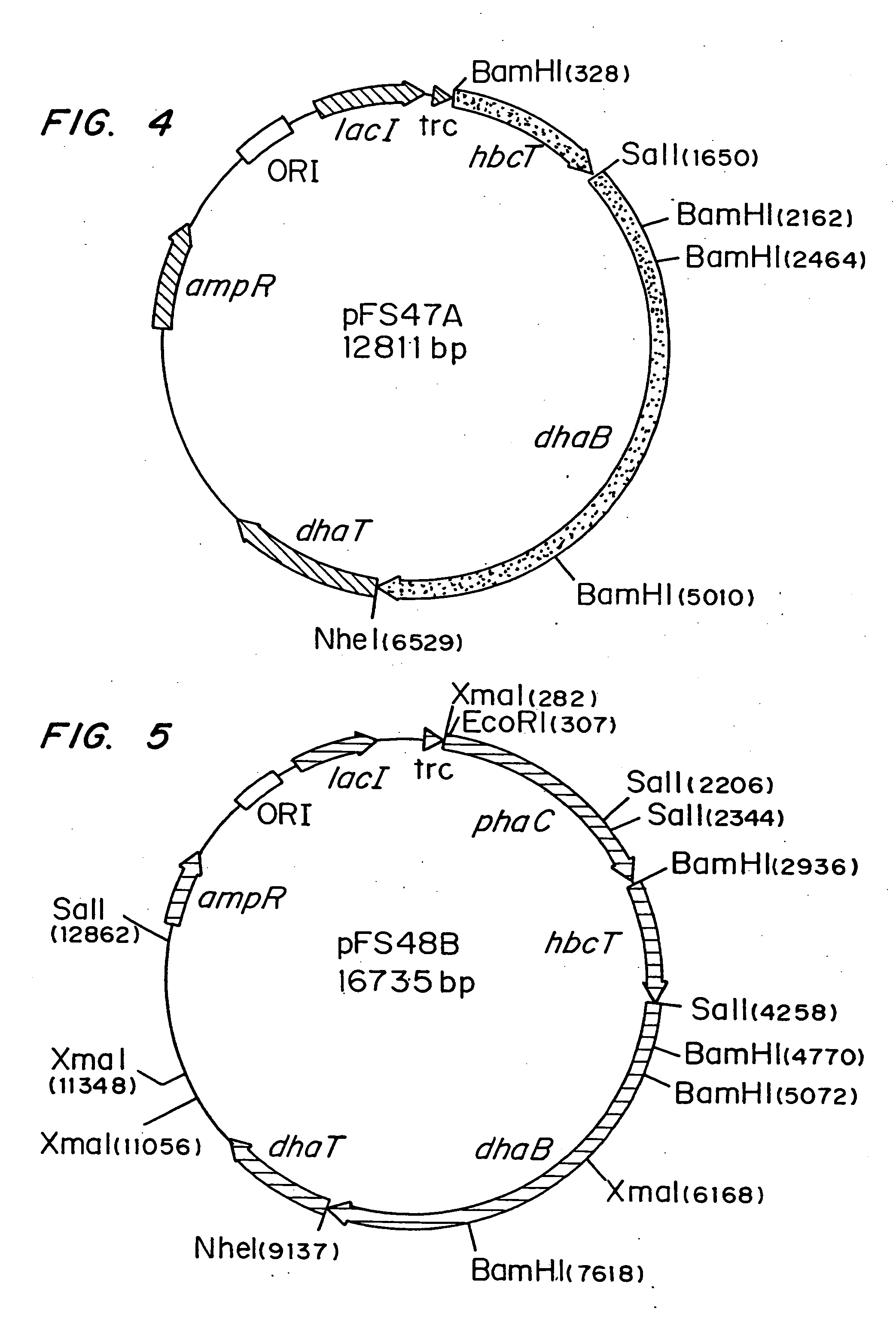
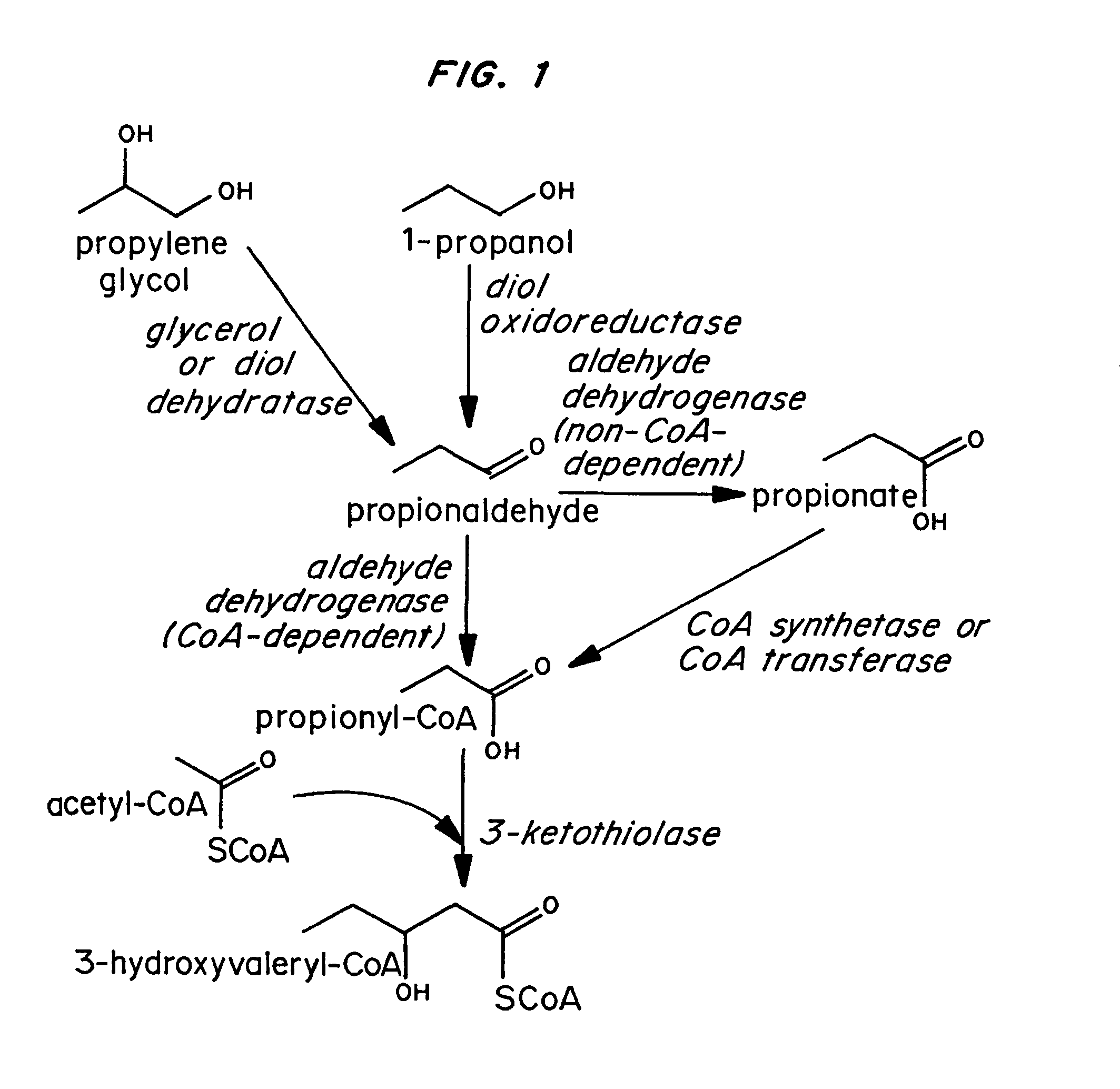
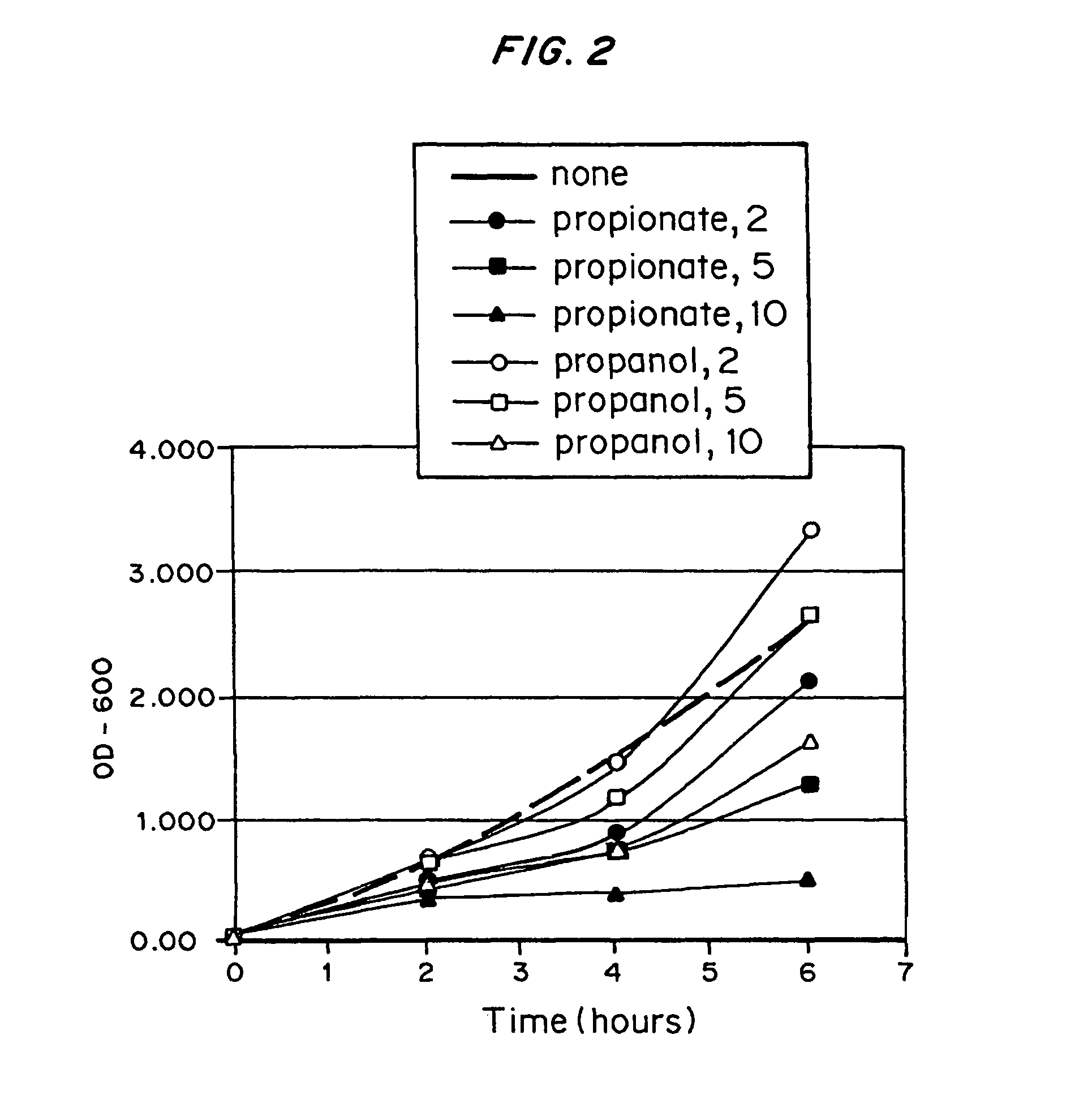
Polyhydroxyalkanoate production from polyols
InactiveUS20050239179A1Promote recoveryProduct can be usedBacteriaMicroorganism based processes3-Hydroxypropionic acidPropanoic acid
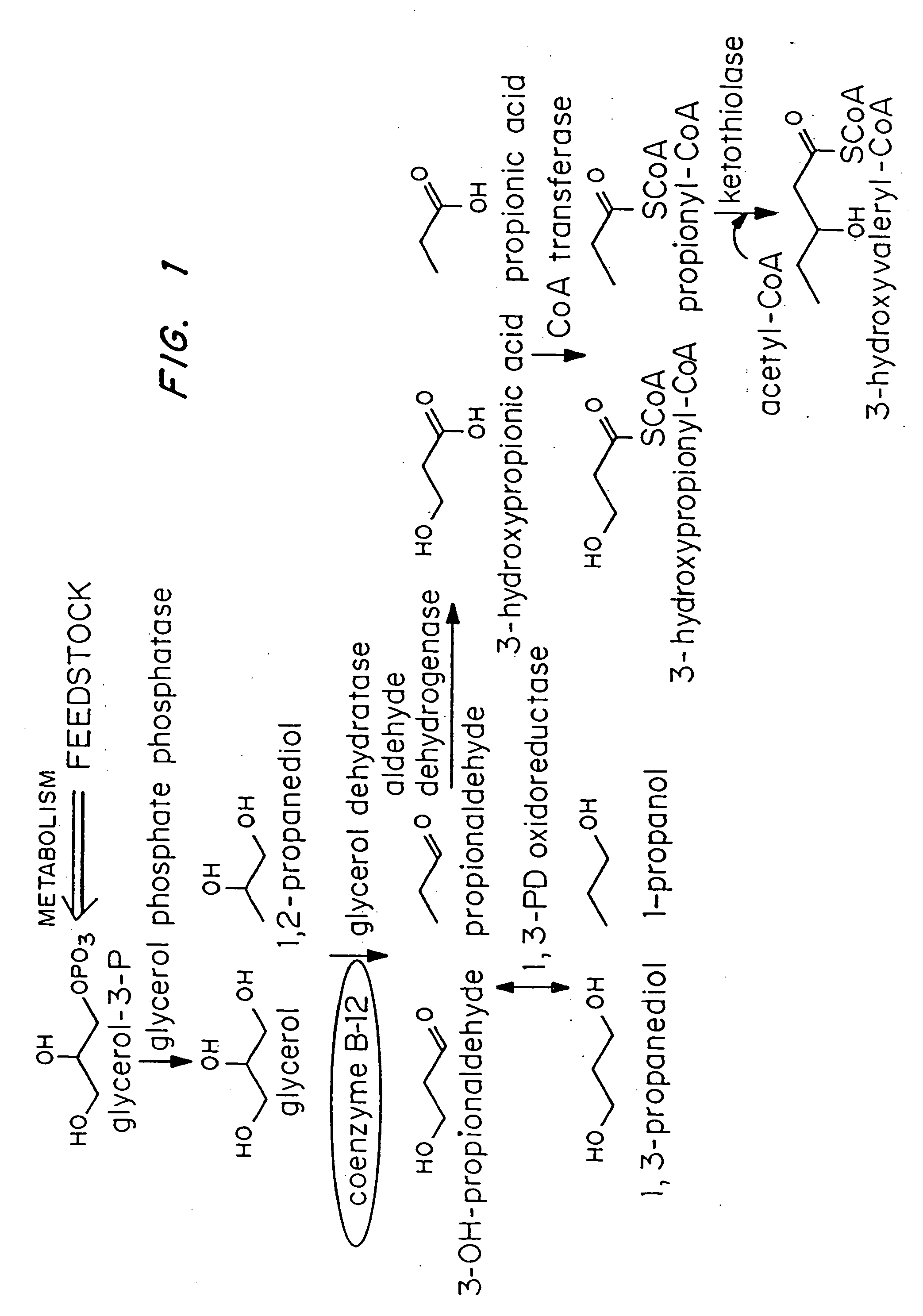
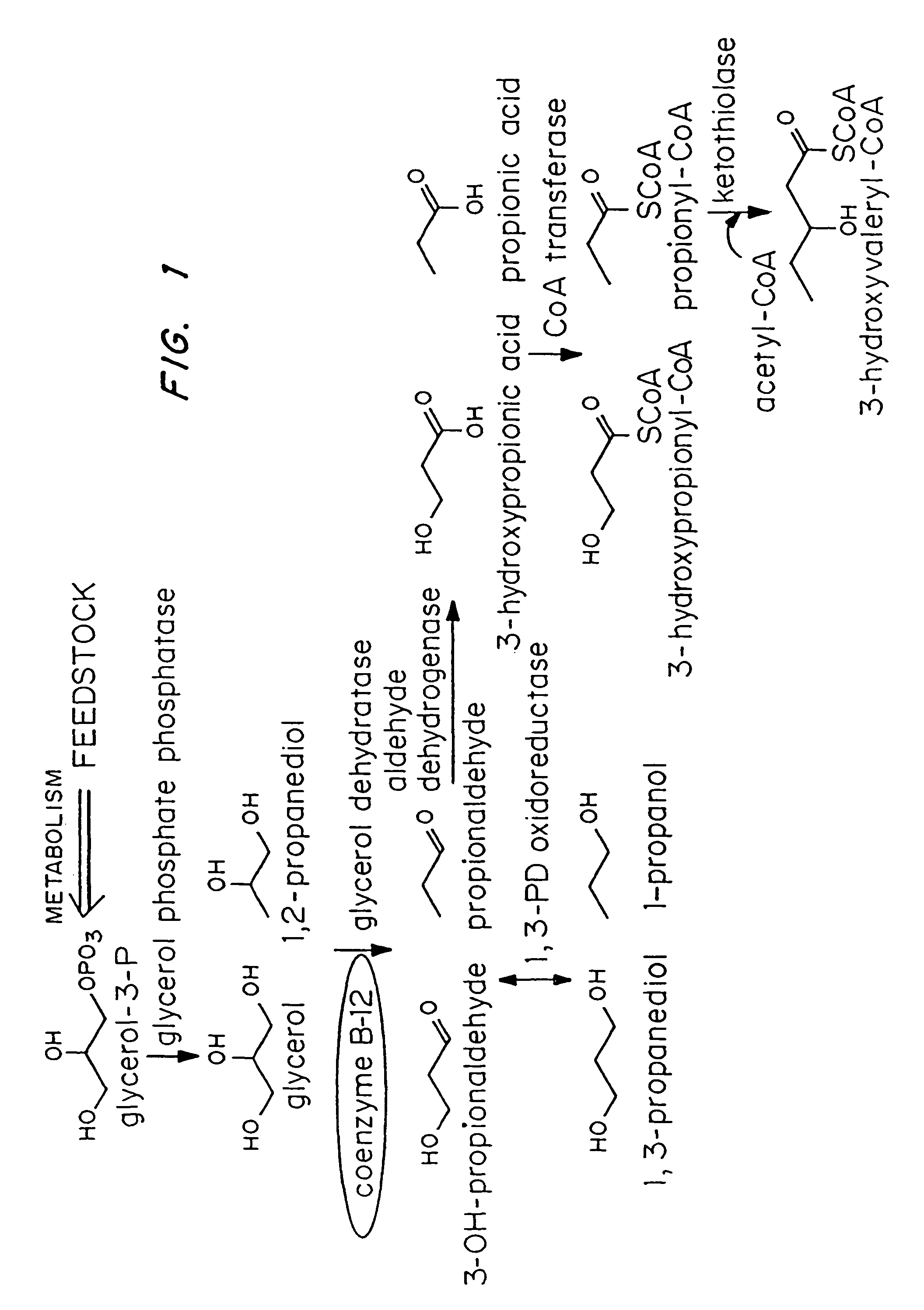
Organisms are provided which express enzymes such as glycerol dehydratase, diol dehydratase, acyl-CoA transferase, acyl-CoA synthetase β-ketothiolase, acetoacetyl-CoA reductase, PHA synthase, glycerol-3-phosphate dehydrogenase and glycerol-3-phosphatase, which are useful for the production of PHAs. In some cases one or more of these genes are native to the host organism and the remainder are provided from transgenes. These organisms produce poly (3-hydroxyalkanoate) homopolymers or co-polymers incorporating 3-hydroxypropionate or 3-hydroxyvalerate monomers wherein the 3-hydroxypropionate and 3-hydroxyvalreate units are derived from the enzyme catalysed conversion of diols. Suitable diols that can be used include 1,2-propanediol, 1,3 propanediol and glycerol. Biochemical pathways for obtaining the glycerol from normal cellular metabolites are also described. The PHA polymers are readily recovered and industrially useful as polymers or as starting materials for a range of chemical intermediates including 1,3-propanediol, 3-hydroxypropionaldehyde, acrylics, malonic acid, esters and amines.
Owner:CJ CHEILJEDANG CORP
Biological systems for manufacture of polyhydroxyalkanoate polymers containing 4-hydroxyacids
The gene encoding a 4-hydroxybutyryl-CoA transferase has been isolated from bacteria and integrated into the genome of bacteria also expressing a polyhydroxyalkanoate synthase, to yield an improved production process for 4HB-containing polyhydroxyalkanoates using transgenic organisms, including both bacteria and plants. The new pathways provide means for producing 4HB containing PHAs from cheap carbon sources such as sugars and fatty acids, in high yields, which are stable. Useful strains are obtaining by screening strains having integrated into their genomes a gene encoding a 4HB-CoA transferase and / or PHA synthase, for polymer production. Processes for polymer production use recombinant systems that can utilize cheap substrates. Systems are provided which can utilize amino acid degradation pathways, α-ketoglutarate, or succinate as substrate.
Owner:CJ CHEILJEDANG CORP
Biological systems for manufacture of polyhydroxyalkanoate polymers containing 4-hydroxyacids
InactiveUS20060084155A1Increase productionHigh activitySugar derivativesBacteriaBiotechnologyDegradation pathway
The gene encoding a 4-hydroxybutyryl-Co A transferase has been isolated from bacteria and integrated into the genome of bacteria also expressing a polyhydroxyalkanoate synthase, to yield an improved production process for 4HB-containing polyhydroxyalkanoates using transgenic organisms, including both bacteria and plants. The new pathways provide means for producing 4HB containing PHAs from cheap carbon sources such as sugars and fatty acids, in high yields, which are stable. Useful strains are obtaining by screening strains having integrated into their genomes a gene encoding a 4HB-CoA transferase and / or PHA synthase, for polymer production. Processes for polymer production use recombinant systems that can utilize cheap substrates. Systems are provided which can utilize amino acid degradation pathways, α-ketoglutarate, or succinate as substrate.
Owner:CJ CHEILJEDANG CORP
Polyhydroxyalkanoate production from polyols
Organisms are provided which express enzymes such as glycerol dehydratase, diol dehydratase, acyl-CoA transferase, acyl-CoA synthetase β-ketothiolase, acetoacetyl-CoA reductase, PHA synthase, glycerol-3-phosphate dehydrogenase and glycerol-3-phosphatase, which are useful for the production of PHAs. In some cases one or more of these genes are native to the host organism and the remainder are provided from transgenes. These organisms produce poly (3-hydroxyalkanoate) homopolymers or co-polymers incorporating 3-hydroxypropionate or 3-hydroxyvalerate monomers wherein the 3-hydroxypropionate and 3-hydroxyvalerate units are derived from the enzyme catalysed conversion of diols. Suitable diols that can be used include 1,2-propanediol, 1,3 propanediol and glycerol. Biochemical pathways for obtaining the glycerol from normal cellular metabolites are also described. The PHA polymers are readily recovered and industrially useful as polymers or as starting materials for a range of chemical intermediates including 1,3-propanediol, 3-hydroxypropionaldehyde, acrylics, malonic acid, esters and amines.
Owner:CJ CHEILJEDANG CORP
Engineered strain of escherichia coli for production of poly-r-3-hydroxyalkanoate polymers with defined monomer unit composition and methods based thereon
Methods and systems for producing prescribed unit size poly(3-hydroxyalkanoate) (PHA) polymers and copolymers are provided. The methods and systems can employ recombinant bacteria that are not native producers of PHA or lack enzymes to degrade PHA once synthesized, metabolize short to long chain fatty acids without induction, and express an (R)-specific enoyl-CoA hydratase and a PHA synthase, the (R)-specific enoyl-CoA hydratase and PHA synthase having wide substrate specificities. The recombinant bacteria are fed at least one fatty acid substrate that is equal in carbon length to the prescribed or desired unit size of the PHA polymer to be produced. The prescribed unit size PHA that is produced is then isolated and / or purified.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
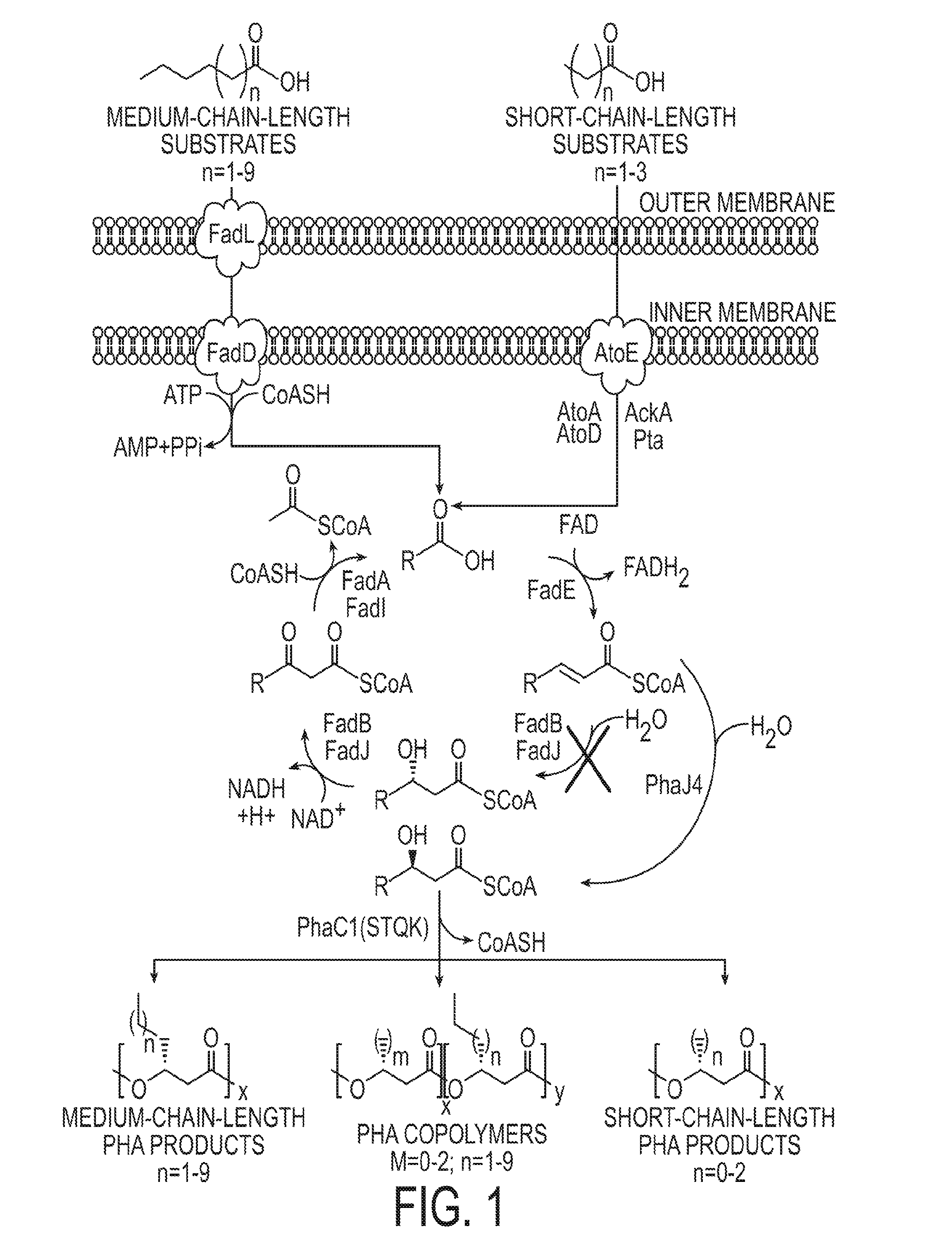
Production of medium chain length polyhydroxyalkanoates from fatty acid biosynthetic pathways
InactiveUS7786355B2Improve the level ofBacteriaHydrolasesPeroxisomal enzymeAcyl Coenzyme A Synthetases
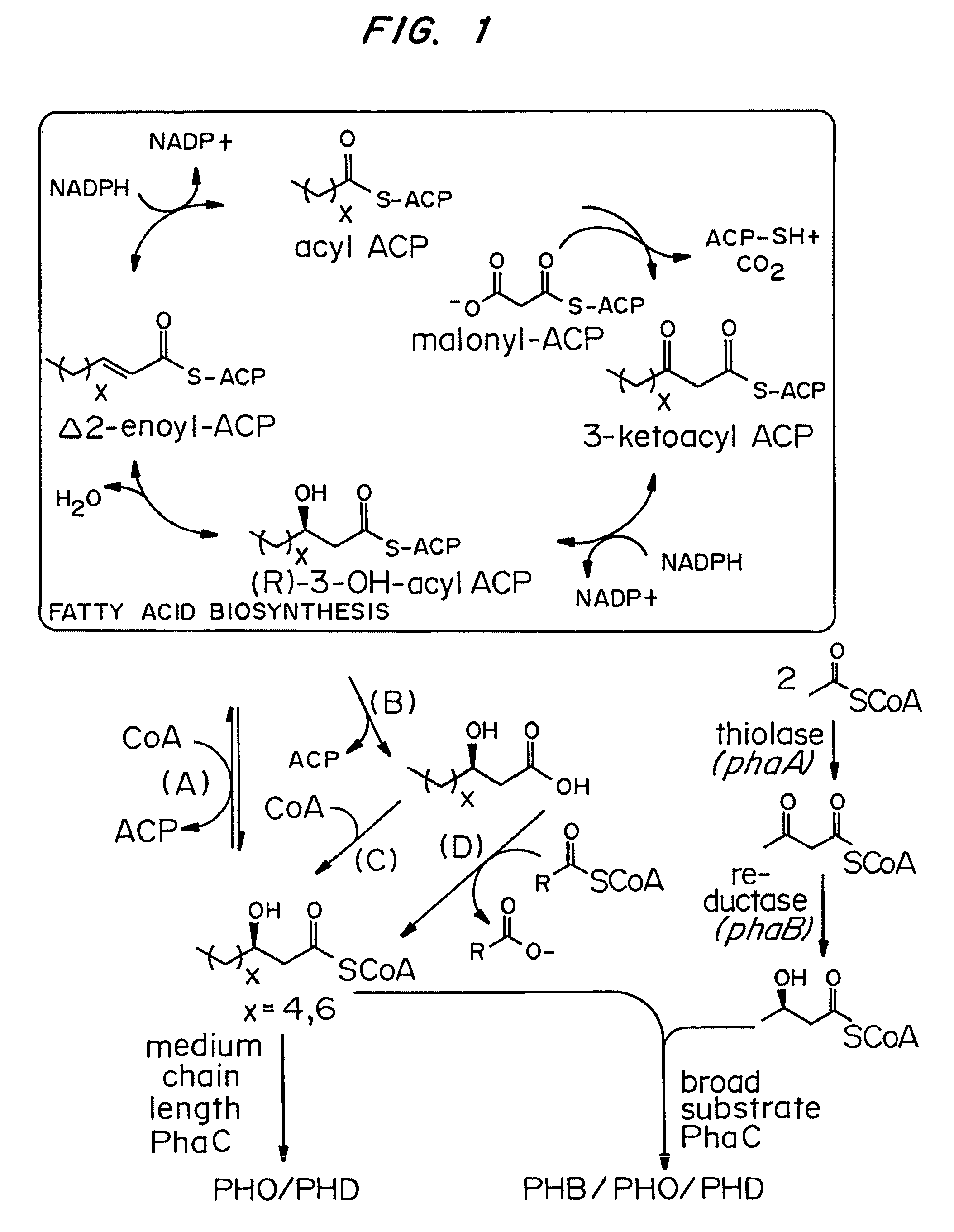
Methods for producing polyhydroxyalkanoates (PHAs) from fatty acid biosynthetic pathways using a 3-hydroxy acyl ACP thioesterase, a PHA synthase, and an acyl CoA synthetase, have been developed. Methodology for enabling PHA production from fatty acid biosynthetic pathways in non-native bacterial PHA producers and plants using an enzyme having the catalytic activity of 3-hydroxy acyl ACP thioesterase, an acyl CoA synthetase with substrate specificity for medium chain length 3-hydroxy fatty acids, and a medium chain length PHA synthase, has been developed. Acyl CoA synthetase activity can be supplied either by the endogenous acyl CoA synthetase of the host organism, when sufficiently expressed, or the host organism's activity can be supplemented by the expression of a recombinant acyl CoA synthetase gene. New strategies are described for plant based PHA production in the chloroplasts, cytosol, and peroxisomes of biomass crops as well as the plastids, cytosol, and peroxisomes of oil seed crops.
Owner:METABOLIX
Engineering strain and application thereof to production of long-chain 3-hydroxy fatty acid
ActiveCN102952774AIncrease productionHigh purityBacteriaMicroorganism based processesLong chain fatty acidPHA synthase
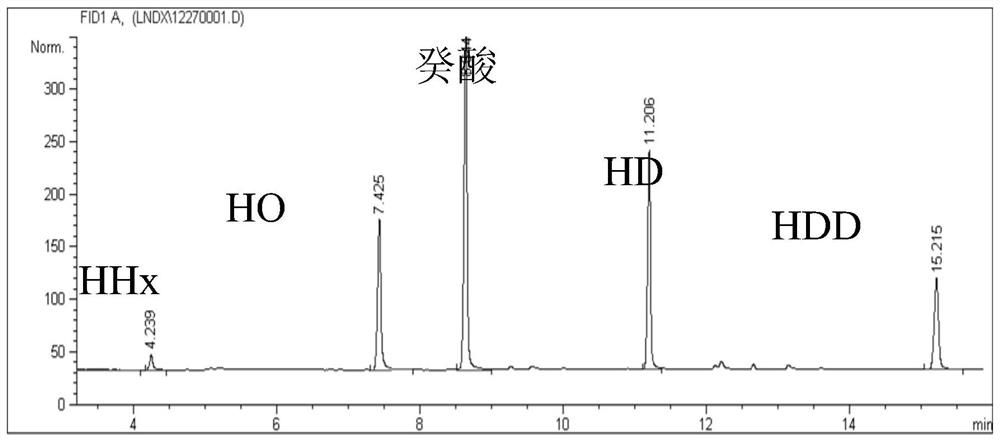
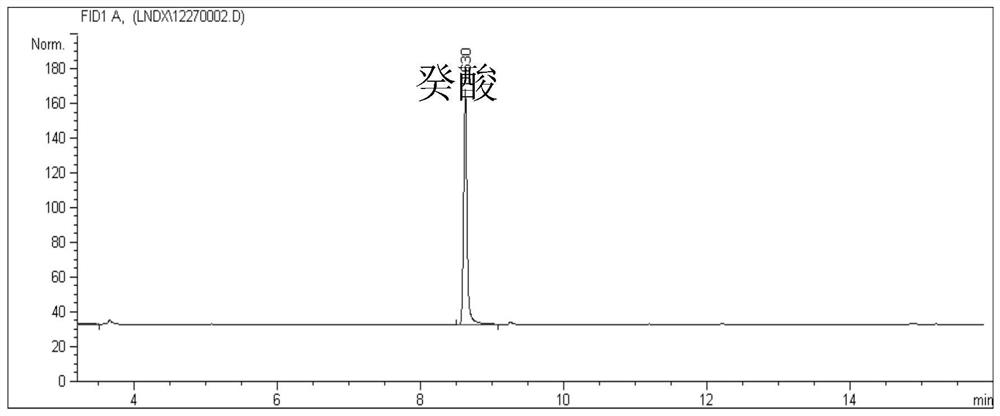
The invention discloses an engineering strain and application thereof to production of long-chain 3-hydroxy fatty acid. The engineering strain provided by the invention is a recombinant strain obtained by conducting the following modification on a starting strain: (a) inactivation of a PHA synthase gene; and (b) introduction of a thioesterase gene. The starting strain is bacteria producing polyhydroxyalkanoates. hen the engineering strain provided by the invention is used, and a middle to long-chain fatty acid is used as a single carbon source, a 3-hydroxy fatty acid product with high yield, high purity, and a structure highly consistent with that of the provided fatty acid carbon source can be obtained in a fermentation liquid. The method provided by the invention can be used for production of 3-hydroxy fatty acid with high yield and high purity, and has wide application prospect.
Owner:TSINGHUA UNIV
Extremely halophilic archaea polyhydroxy fatty acid ester synthases and encoding gene and application
InactiveCN101139575AHigh PHA synthase activityIncreased PHA synthase activityBacteriaEnzymesEnzyme GenePHA synthase
The invention discloses an extremely halophilic archaea polyhydroxyalkanoates enzyme and the code gene and application of the enzyme. The polyhydroxyalkanoates enzyme comprises PhaEHh subunit and PhaCHh subunit; the PhaEHh subunit has amino acid residue sequence in sequence 1 in the sequence table or amino acid residue sequence got by substituting and / or deleting and / or adding one or more amino acid residue for the amino acid residue sequence in sequence 1 in the sequence table; the PhaCHh subunit has amino acid residue sequence in sequence 2 in the sequence table or amino acid residue sequence got by substituting and / or deleting and / or adding one or more amino acid residue for the amino acid residue sequence in sequence 2 in the sequence table. The polyhydroxyalkanoates enzyme is of very high PHA enzyme activity; introducing the polyhydroxyalkanoates enzyme gene phaECHh into a host bacterial strain will make possible high-efficient expression of PHA enzyme, and can improve the activity of the extremely halophilic archaea polyhydroxyalkanoates enzyme and PHA output.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Pha-producing microorganism having sucrose assimilability, and method for producing pha using said microorganism
An object of the present invention is to provide a PHA-producing microorganism which can assimilate sucrose, and a method for producing a PHA by culturing this microorganism, using sucrose as a carbon source. A PHA-producing microorganism, comprising a PHA synthase gene, and heterogeneous-organism-derived genes in the following items (1) and (2): (1) a sucrose hydrolase gene encoding an amino acid sequence described in SEQ ID NO: 1, or a gene encoding a polypeptide which has a sequence homology of 90% or more to the amino acid sequence and which has sucrose hydrolase activity; and (2) a sucrose permease gene encoding an amino acid sequence described in SEQ ID NO: 2, or a gene encoding a polypeptide which has a sequence homology of 90% or more to the amino acid sequence and which has sucrose permease activity. The invention is also a method for producing a PHA, including the step of culturing this microorganism in a medium including sucrose as a carbon source.
Owner:KANEKA CORP
Microorganism capable of producing polyhydroxyalkanoate, polyhydroxyalkanoate synthase, and gene encoding the same
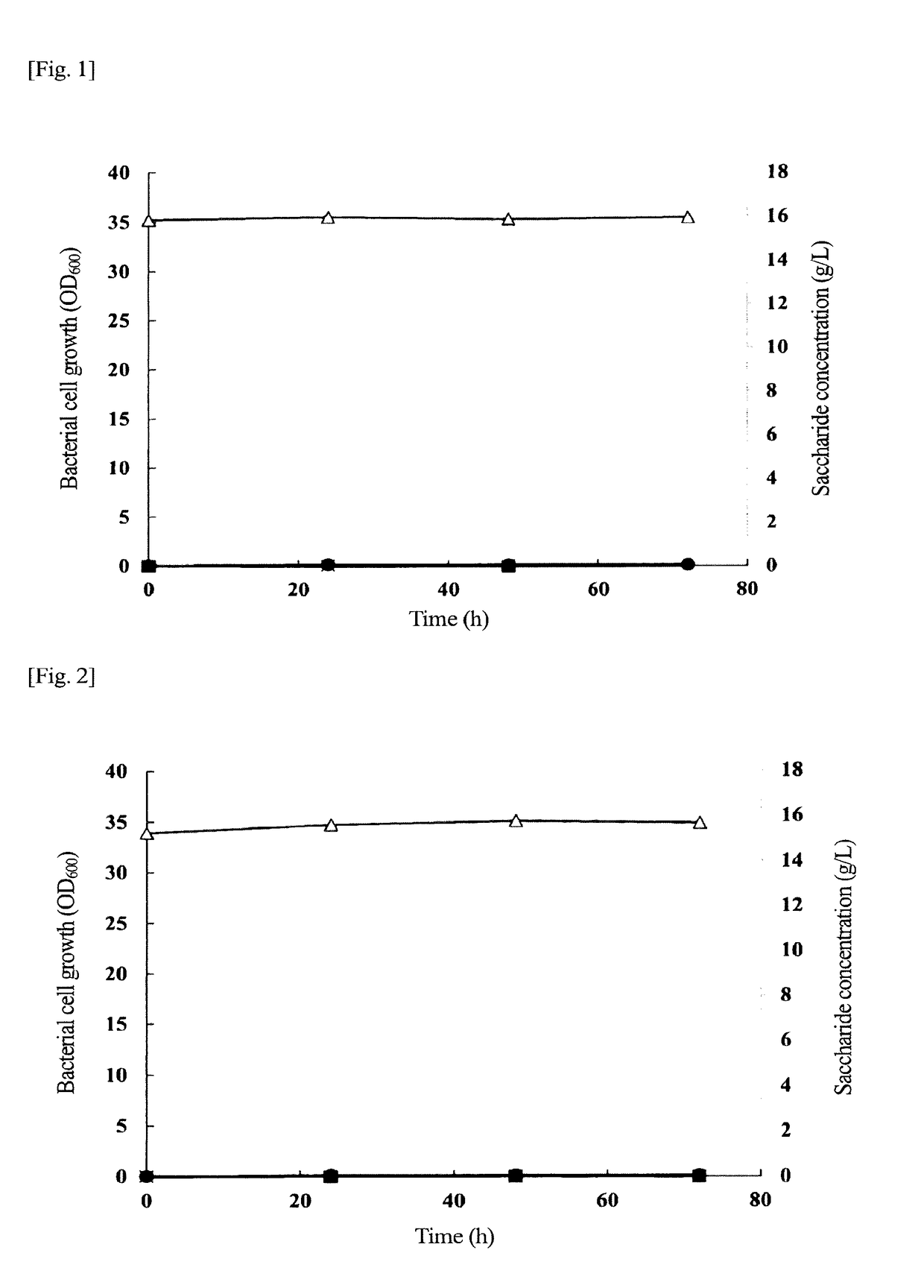
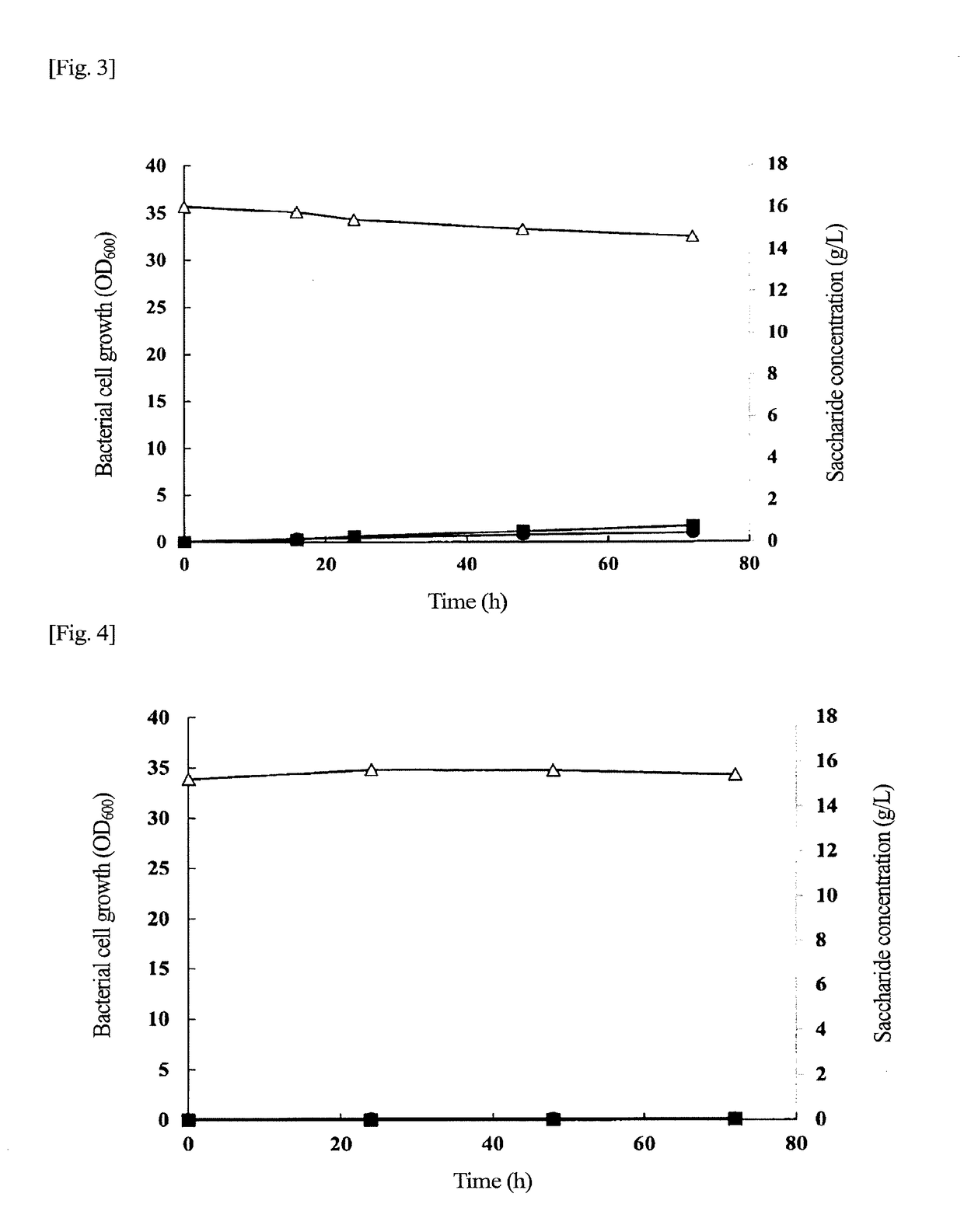
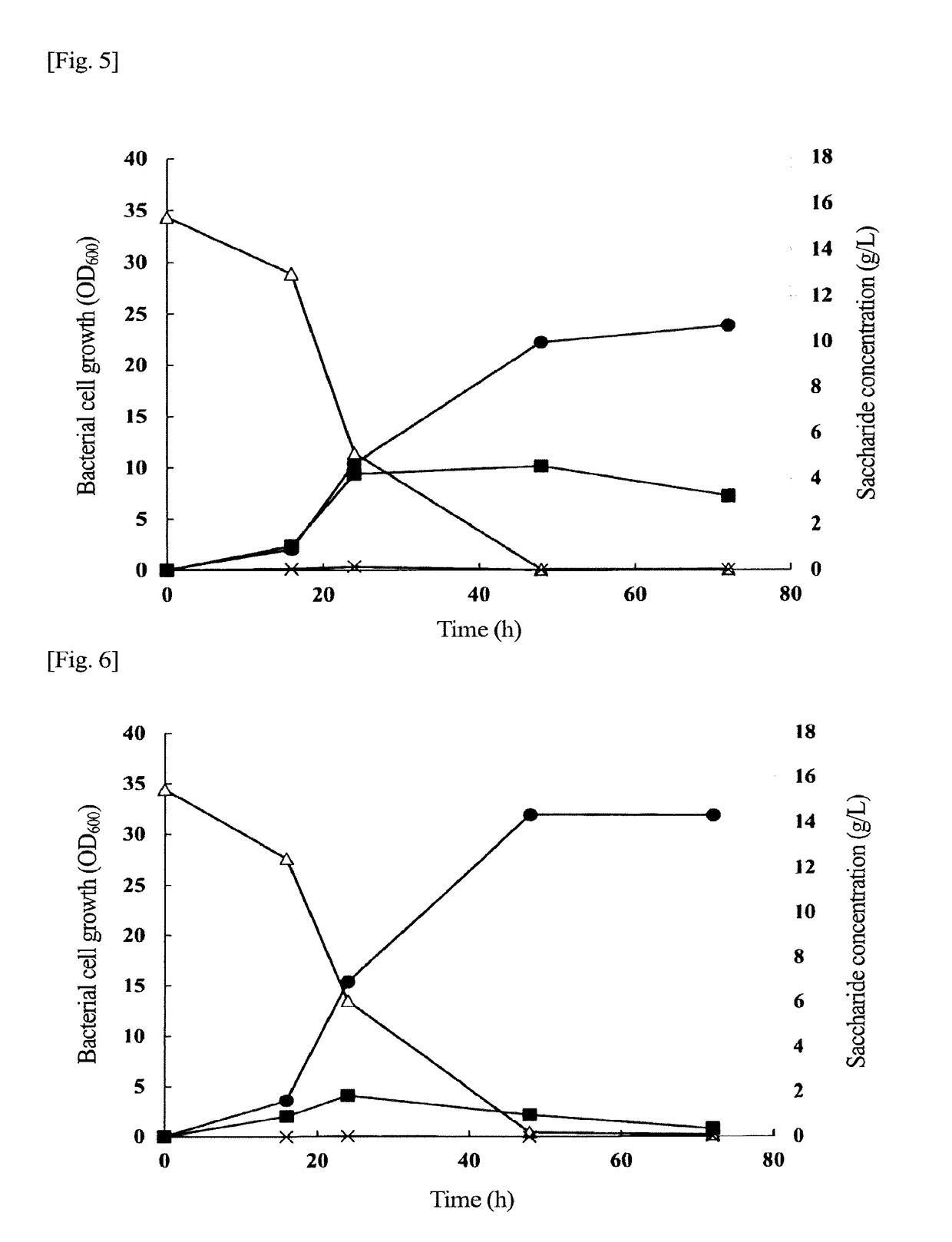
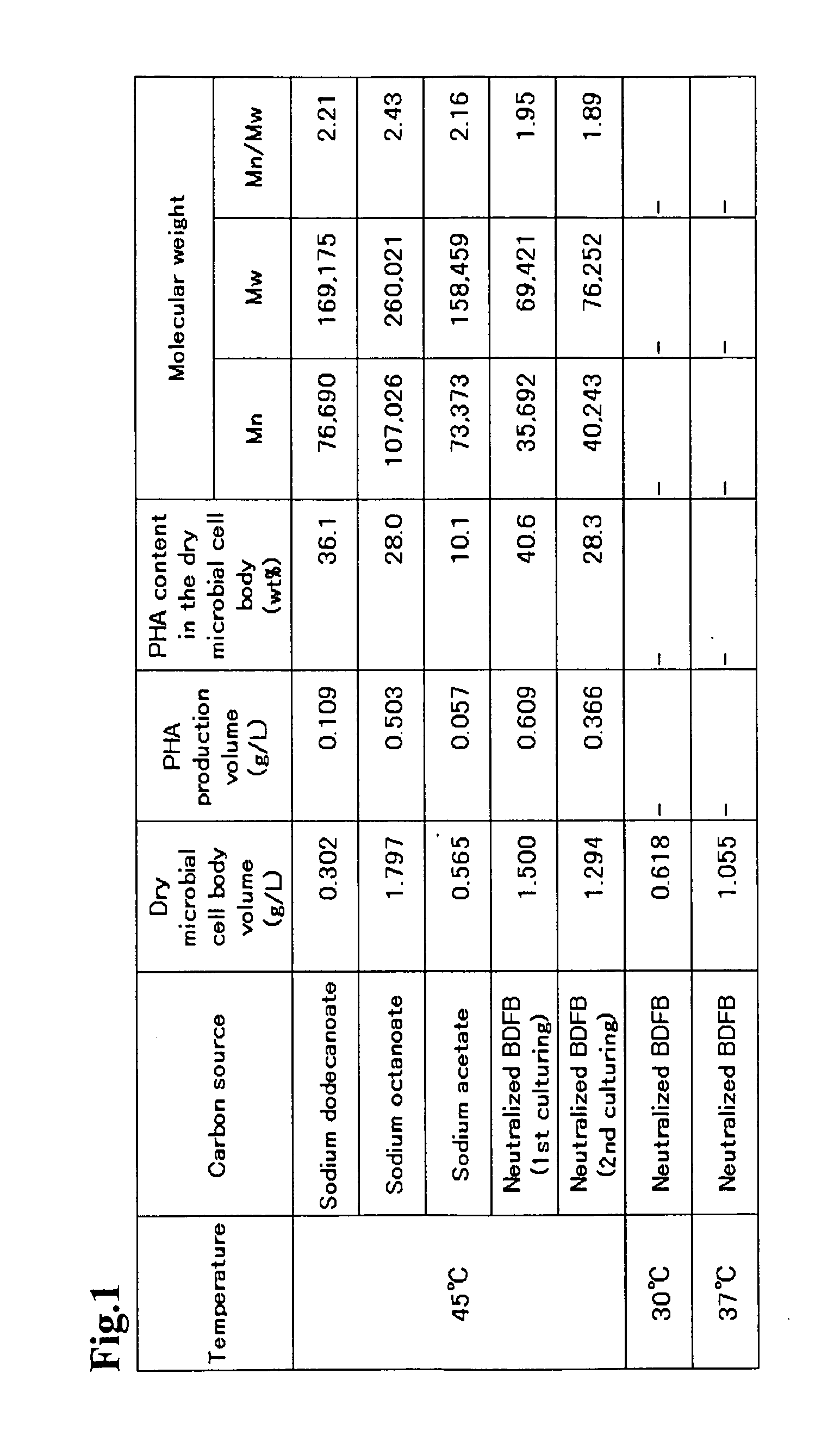
Problem to be Solved: To provide a new microorganism capable of producing a polyhydroxyalkanoate (PHA), a PHA synthase gene, an expression cassette including the gene, a vector including the expression cassette, a transformant transformed by the vector, a polypeptide having PHA synthase activity, a method for producing a PHA synthase and a method for producing a PHA.Solution: The new microorganism is capable of producing a polyhydroxyalkanoate comprising a 16S rRNA gene whose polynucleotide sequence shows 99% or more homology to a polynucleotide sequence represented by SEQ ID No: 1, having an optimum temperature of an activity temperature range for the growth and PHA production of the microorganism of at least 45° C. and being capable of growing at a pH range from 6 to 10.
Owner:HITACHI CHEM CO LTD
Process for prepn. of polyhydroxy alkanoate by using maoC gene
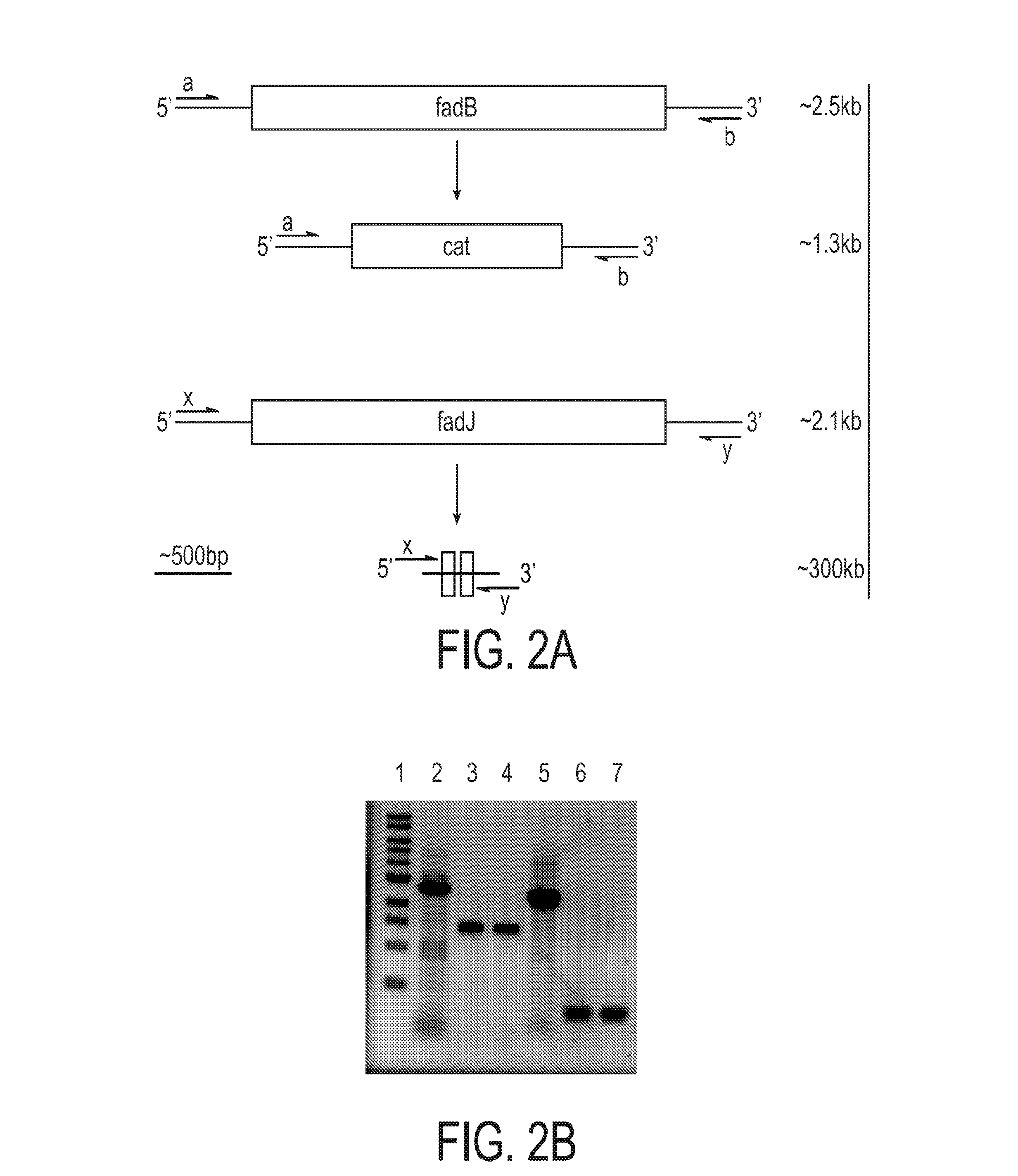
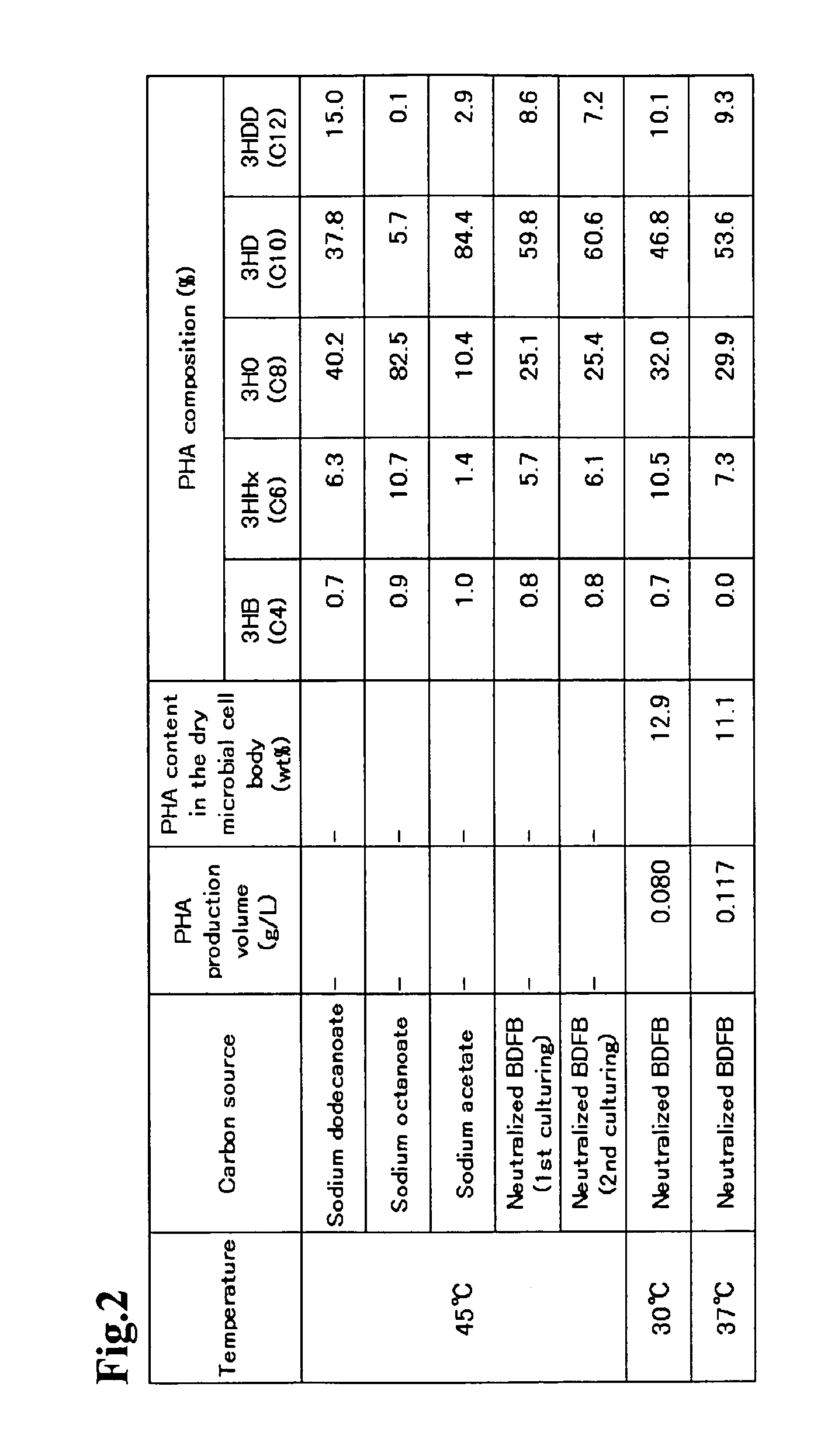
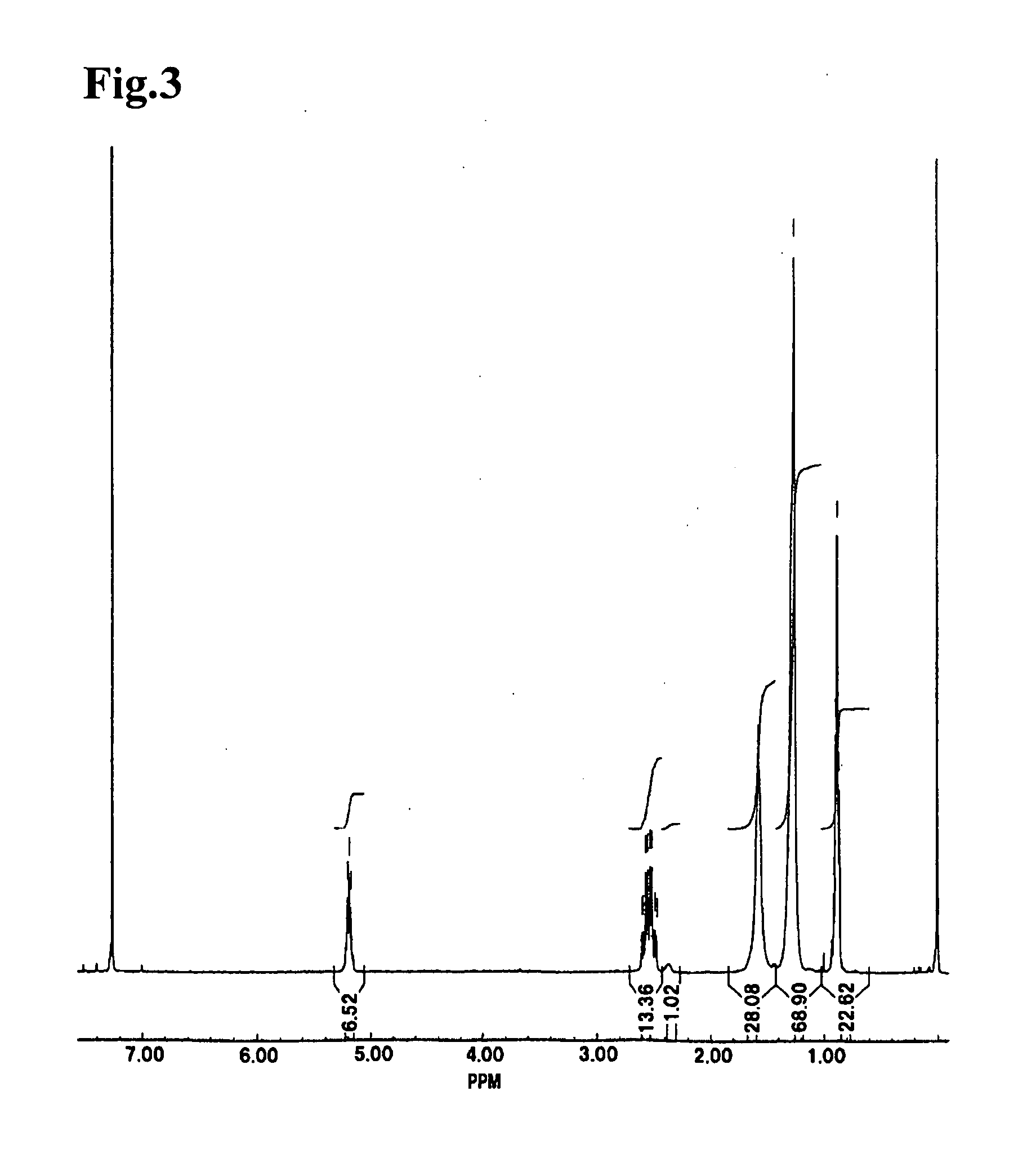
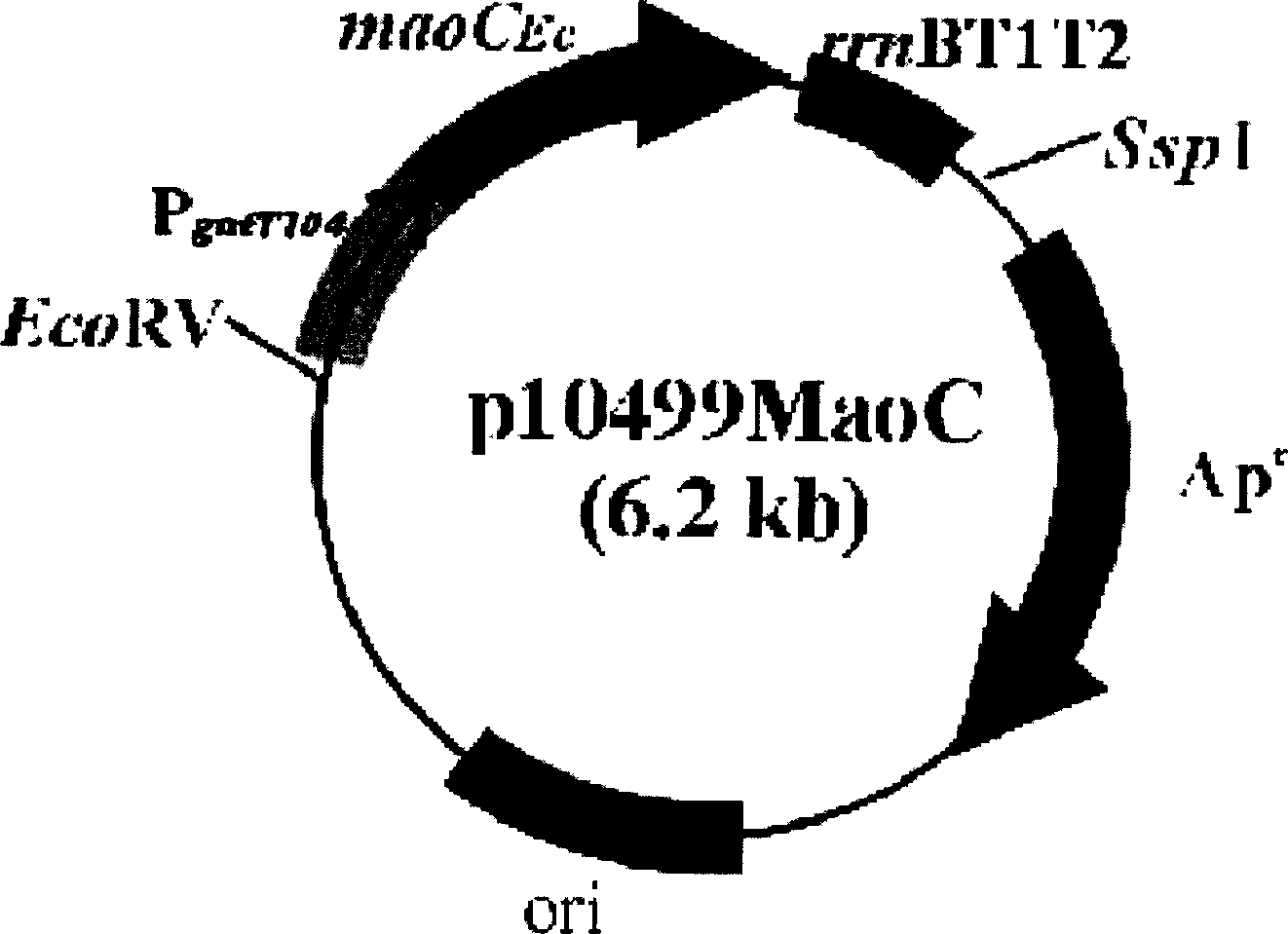
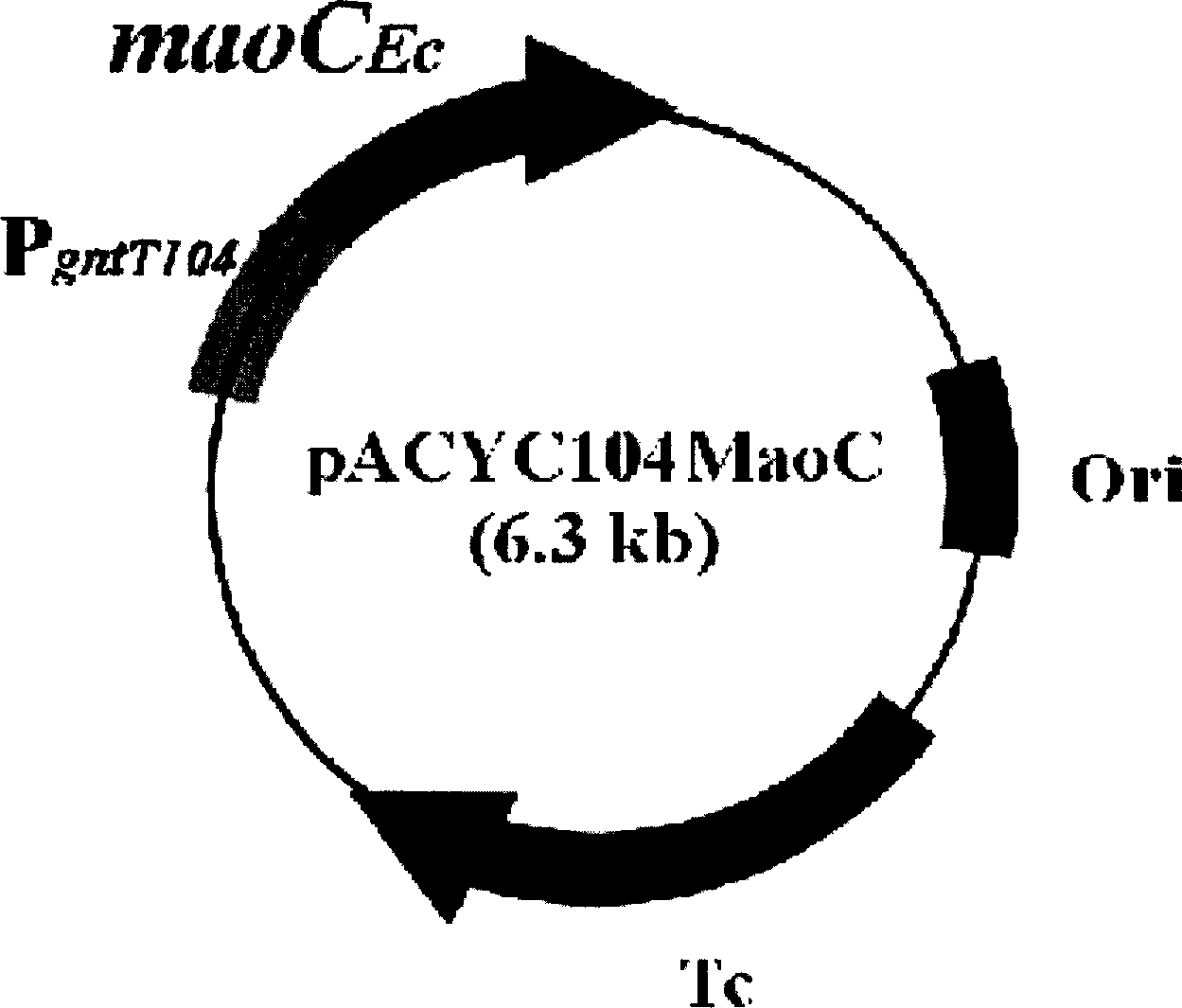
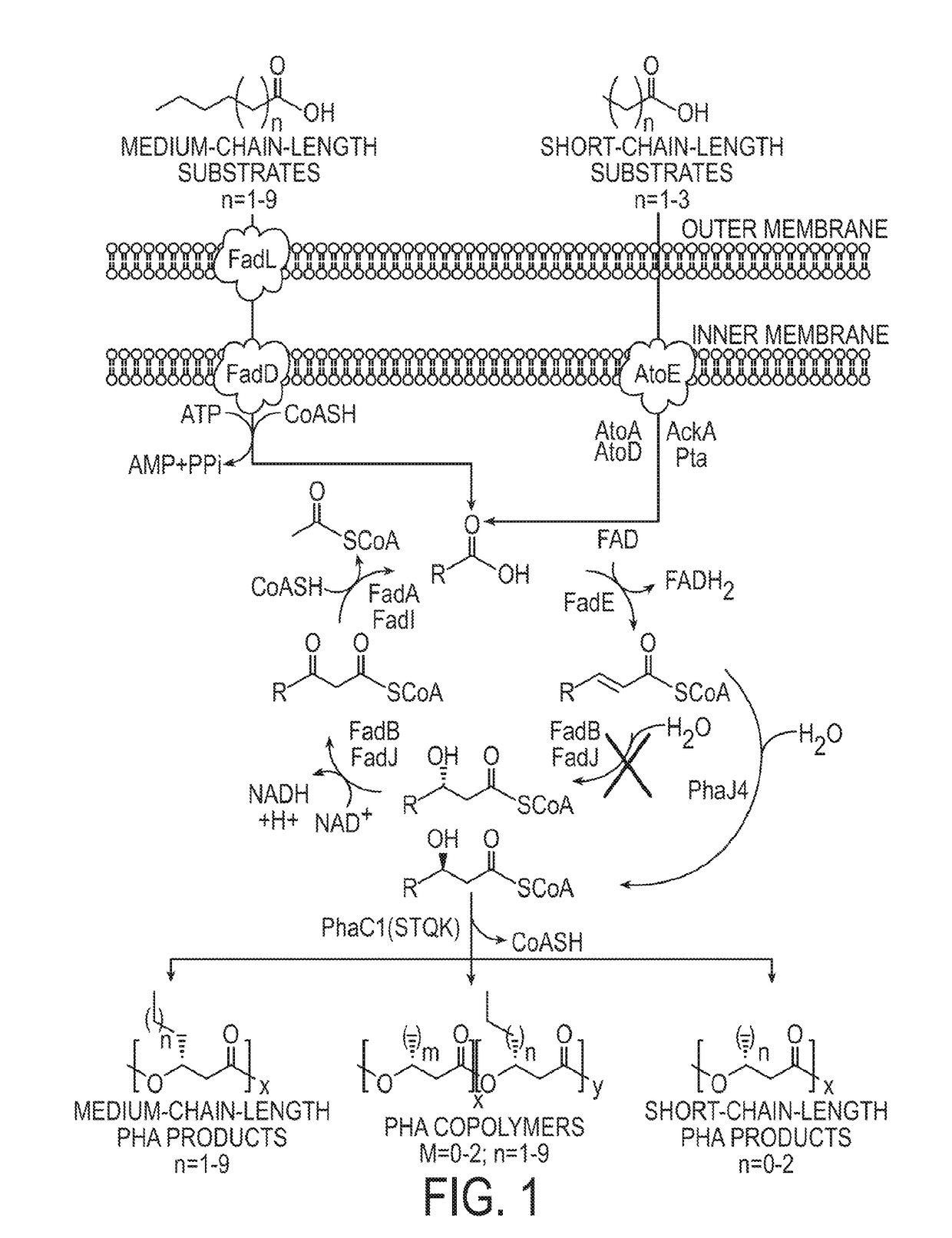
The present invention relates to a method for producing middle-chain-length polyhydroxyalkanoate (MCL-PHA) using a maoC gene. The producing method of MCL-PHA according to the present invention comprises the steps of transforming a microorganism with the maoC gene to give a transformant, the microorganism being deleted of a fadB gene and containing a PHA synthase gene; culturing the transformant in medium containing a C6-10 carbon source; and obtaining PHA consisting of monomers with 6-10 carbon atoms. When the maoC gene whose function has not yet been established is used according to the present invention, high quality PHA with a higher number of carbon atoms than the prior PHA can be produced at a higher efficiency.
Owner:KOREA ADVANCED INST OF SCI & TECH
A kind of engineering bacteria and its application in the production of medium and long-chain 3-hydroxy fatty acids
The invention discloses an engineering strain and application thereof to production of long-chain 3-hydroxy fatty acid. The engineering strain provided by the invention is a recombinant strain obtained by conducting the following modification on a starting strain: (a) inactivation of a PHA synthase gene; and (b) introduction of a thioesterase gene. The starting strain is bacteria producing polyhydroxyalkanoates. hen the engineering strain provided by the invention is used, and a middle to long-chain fatty acid is used as a single carbon source, a 3-hydroxy fatty acid product with high yield, high purity, and a structure highly consistent with that of the provided fatty acid carbon source can be obtained in a fermentation liquid. The method provided by the invention can be used for production of 3-hydroxy fatty acid with high yield and high purity, and has wide application prospect.
Owner:TSINGHUA UNIV
Poly(3-hydroxypropionate-b-lactate) block copolymer using microorganisms
The present invention relates to a novel 3-hydroxypropionate-lactate block copolymer [P(3HP-b-LA)], and a method for preparing same, and more specifically, provides a method for preparing a 3-hydroxypropionate-lactate block copolymer, and a 3-hydroxypropionate-lactate block copolymer prepared thereby, the method comprising: a first culture step in which, by using recombinant E. coli enhanced so asto be incapable of biosynthesizing lactic acid, P(3HP) is biosynthesized at the early stage of culturing by having glycerol as a carbon source and through 3-hydroxypropionate-generating genes and anenhanced PHA synthase; and a second culture step in which P(3HP) production is inhibited by using a carbon catabolic repression system for selectively introducing only glucose into E. coli when glycerol and glucose are supplied together as carbon sources, and in which polylactate is biosynthesized to an interrupted P(3HP) terminus by the enabling of the expression of a lactate synthase and a lactyl-CoA converting enzyme through an IPTG induction system.
Owner:LG CHEM LTD
Microorganism having pha synthase-coding genes and method for producing pha using same
ActiveUS20180305722A1Increase heightIncrease speedBacteriaAcyltransferasesGenus AeromonasMicroorganism
An object of the invention is to improve the crystallization speed of a PHA copolymer which is to be slowly crystallized, and improve the melt workability and productivity. A microorganism is used which has genes encoding two or more different PHA synthases derived from the genus Aeromonas. The genes encoding the PHA synthases derived from the genus Aeromonas preferably include genes encoding at least two PHA synthases which are capable of synthesizing a copolymer PHA including, as monomer unit species, 3-hydroxybutyric acid and 3-hydroxyhexanoic acid, and which are different in substrate specificity toward 3-hydroxyhexanoic acid from each other. When this microorganism is cultured, a PHA mixture can be produced which includes three or more PHA species different in melting point from each other.
Owner:KANEKA CORP
Recombinant microorganism and method for producing aliphatic polyester with the use of the same
InactiveUS20120122165A1Large capacityIncrease production capacityBacteriaTransferasesPolyesterMicroorganism
This invention provides a recombinant microorganism exhibiting excellent aliphatic polyester productivity and a method for producing an aliphatic polyester with the use of the recombinant microorganism. In this invention, the Megasphaera elsdenii-derived pct gene (and / or the Staphylococcus aureus-derived pct gene) and the Pseudomonas sp. 61-3 strain-derived PHA synthase gene (phaC2 gene) (and / or the Alcanivorax borkumensis SK2 strain-derived PHA synthase gene) are introduced into a host microorganism.
Owner:TEIJIN LTD
Method for producing a polyhydroxyalkanoate using a microorganism belonging to the genus Cupriavidus
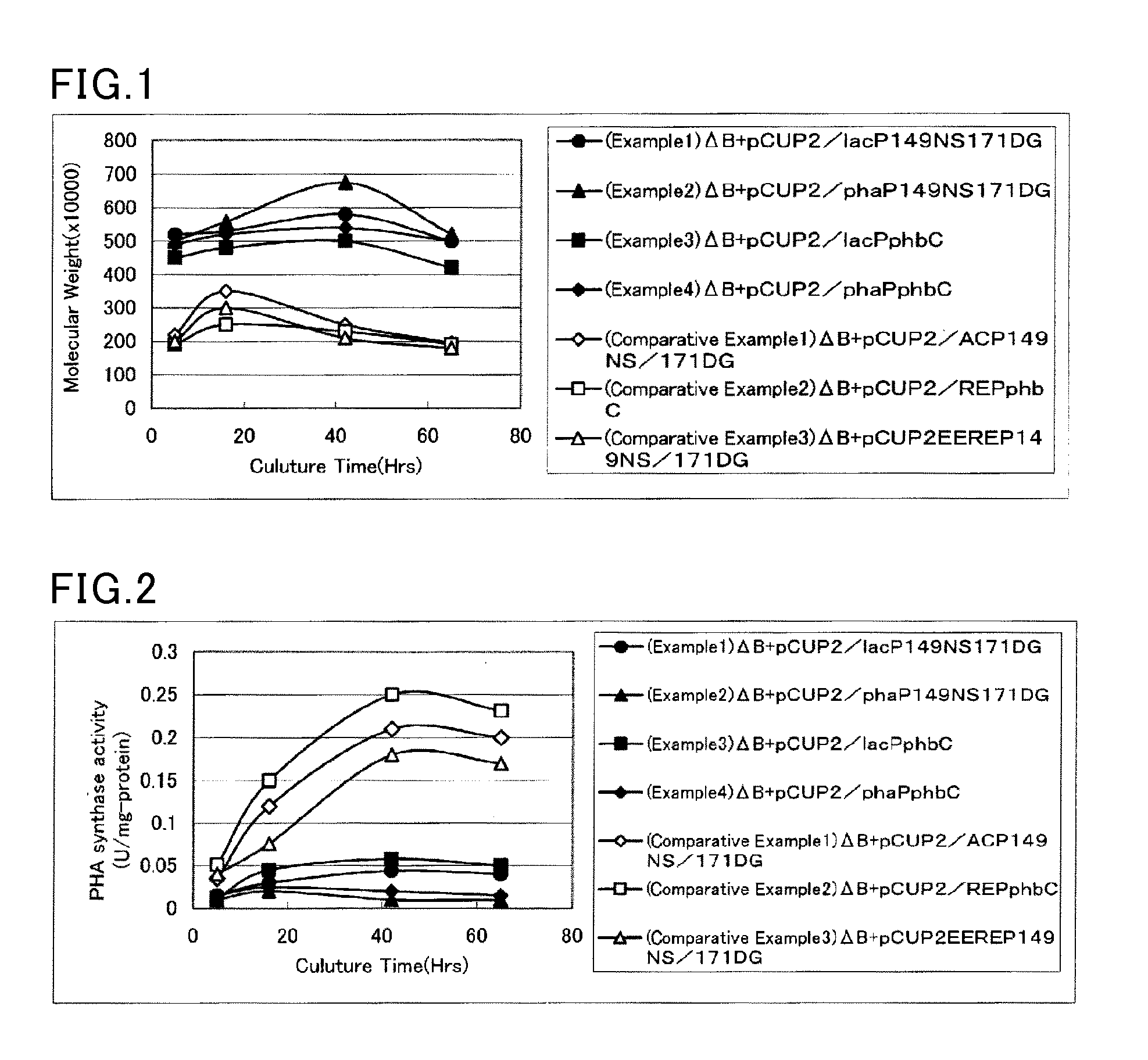
The present invention provides a microorganism that synthesizes a high-molecular-weight PHA, and a method for producing a high-molecular-weight PHA, which have a productivity of at least 100 g / L. The provision is achieved by controlling the specific activity of a PHA synthase in cells of a microorganism that belongs to the genus Cupriavidus and is capable of producing a PHA, to 0.1 U / mg-protein at most. The microorganism and the method enable industrially efficient production of a PHA with a weight average molecular weight of at least 4,000,000.
Owner:KANEKA CORP
Method for polymerising glycolic acid with microorganisms
The present invention relates to a method for producing and preparing polyglycolate (PGA) from genetically engineered organisms. More specifically, the invention relates to a method comprising two steps; 1) culturing, in a medium containing glycolic acid or not, the microorganism expressing at least one gene encoding an enzyme(s) that converts glycolate into glycolyl-CoA, and a gene encoding polyhydroxyalkanoate (PHA) synthase which uses glycolyl-CoA as a substrate, 2) recovering the polyglycolate polymer.
Owner:METABOLIC EXPLORER
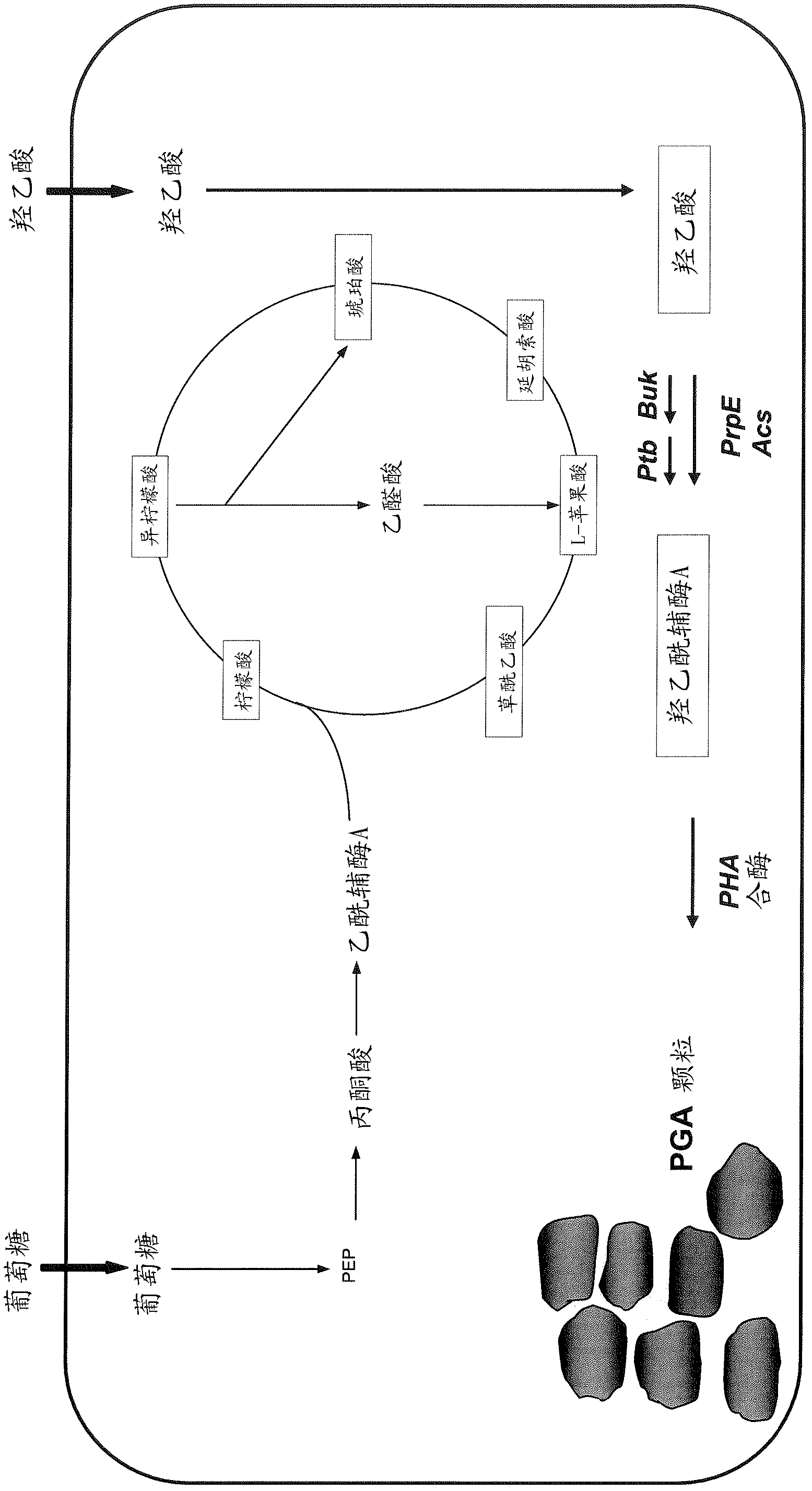
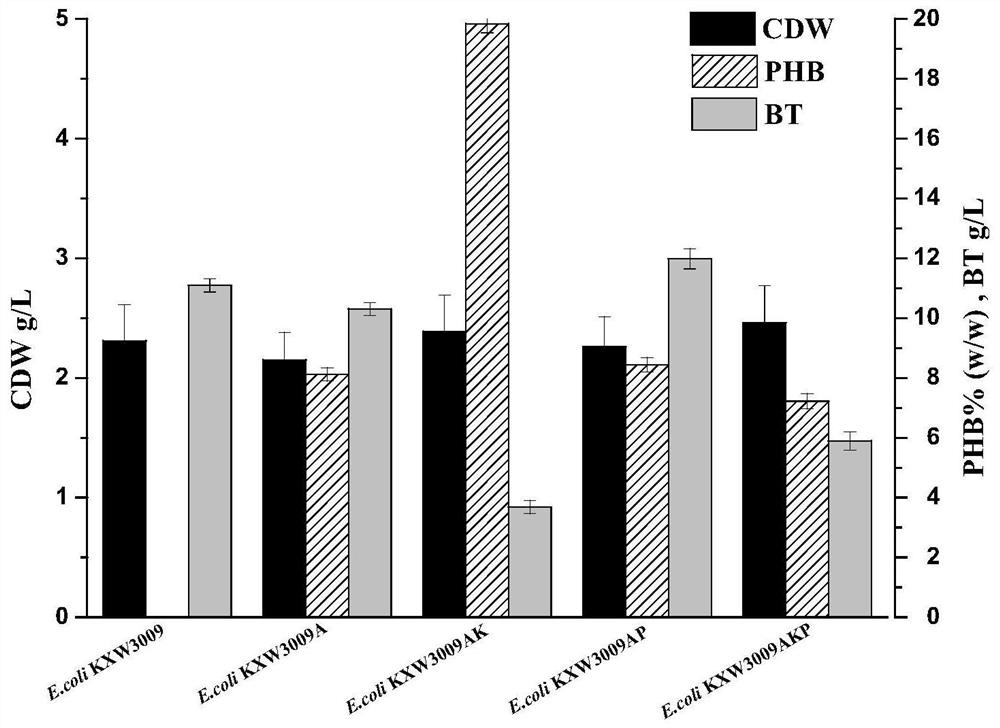
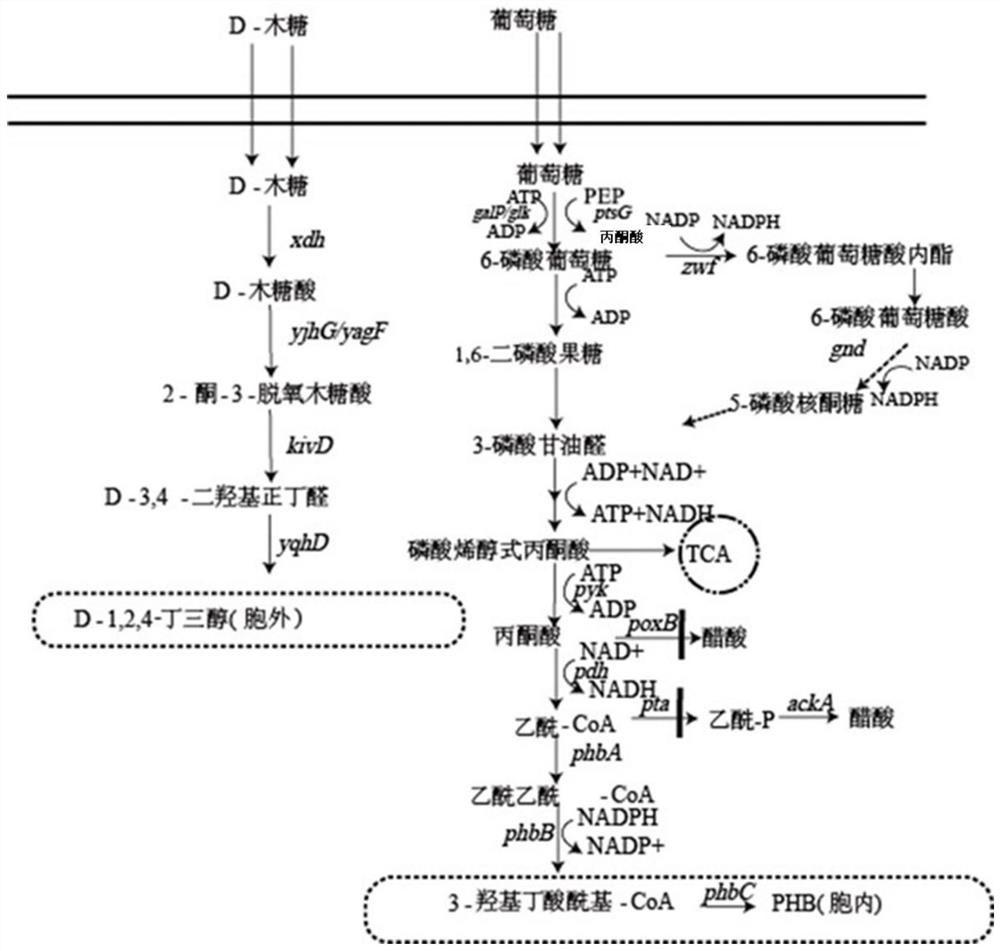
Method for co-production of D-1,2,4-butanetriol and PHB by metabolic modification of escherichia coli, and application
PendingCN112094793AReduce fermentation costsEasy to useBacteriaMicroorganism based processesEscherichia coliPHA synthase
The invention discloses a method for co-production of D-1,2,4-butanetriol and PHB by metabolic modification of escherichia coli, and application, and belongs to the field of gene engineering. According to the invention, a genetic engineering method is applied; and plasmids with over-expression of beta-keto thiolase PhbA, acetoacetyl coenzyme A reductase PhbB and PHA synthase PhbC from alcaligeneseutrophus are transferred into engineering bacteria E.coli KXM3009 to construct a synthetic route of PHB. Furthermore, the gene glk related to glycolysis is over-expressed, so that co-production of 1,2,4-butantriol and PHB is realized; and a foundation is laid for co-production of multiple added-value products.
Owner:JIANGNAN UNIV
Microorganism producing high-molecular-weight pha
The present invention provides a microorganism that synthesizes a high-molecular-weight PHA, and a method for producing a high-molecular-weight PHA, which have a productivity of at least 100 g / L. The provision is achieved by controlling the specific activity of a PHA synthase in cells of a microorganism that belongs to the genus Cupriavidus and is capable of producing a PHA, to 0.1 U / mg-protein at most. The microorganism and the method enable industrially efficient production of a PHA with a weight average molecular weight of at least 4,000,000.
Owner:KANEKA CORP
Engineered strain of escherichia coli for production of poly-r-3-hydroxyalkanoate polymers with defined monomer unit composition and methods based thereon
Methods and systems for producing prescribed unit size poly(3-hydroxyalkanoate) (PHA) polymers and copolymers are provided. The methods and systems can employ recombinant bacteria that are not native producers of PHA or lack enzymes to degrade PHA once synthesized, metabolize short to long chain fatty acids without induction, and express an (R)-specific enoyl-CoA hydratase and a PHA synthase, the (R)-specific enoyl-CoA hydratase and PHA synthase having wide substrate specificities. The recombinant bacteria are fed at least one fatty acid substrate that is equal in carbon length to the prescribed or desired unit size of the PHA polymer to be produced. The prescribed unit size PHA that is produced is then isolated and / or purified.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Polyhydroxyalkanoate production by coenzyme A-dependent aldehyde dehydrogenase pathways
Organisms are provided containing genes encoding one or more enzymes, Coenzyme-A-dependent aldehyde dehydrogenase, acyl-CoA transferase, acyl-CoA synthetase, β-ketothiolase, acetoacetyl-CoA reductase and / or PHA synthase. In some cases one or more of these genes are native to the host organism and the remainder are heterologous genes provided by genetic engineering. These organisms produce poly (3-hydroxyalkanoate) homopolymers or co-polymers comprising 3-hydroxalkanoate monomers other than 3-hydroxybutryrate wherein these 3-hydroxyalkanoate units are derived from the enzyme-catalyzed conversion of alcohols to 3-hydroxyacyl-CoA monomers, where at least one step in the conversion pathway involves a Co-enzyme A-dependent aldehyde dehydrogenase activity. The PHA polymers are readily recovered and industrially useful as polymers for articles such as films, latexes, coatings, adhesives, fibers, binders, resins, and medical devices.
Owner:CJ CHEILJEDANG CORP
Method for producing PHA (polyhydroxyalkanoate) by using pseudomonas corrugata type II synthase
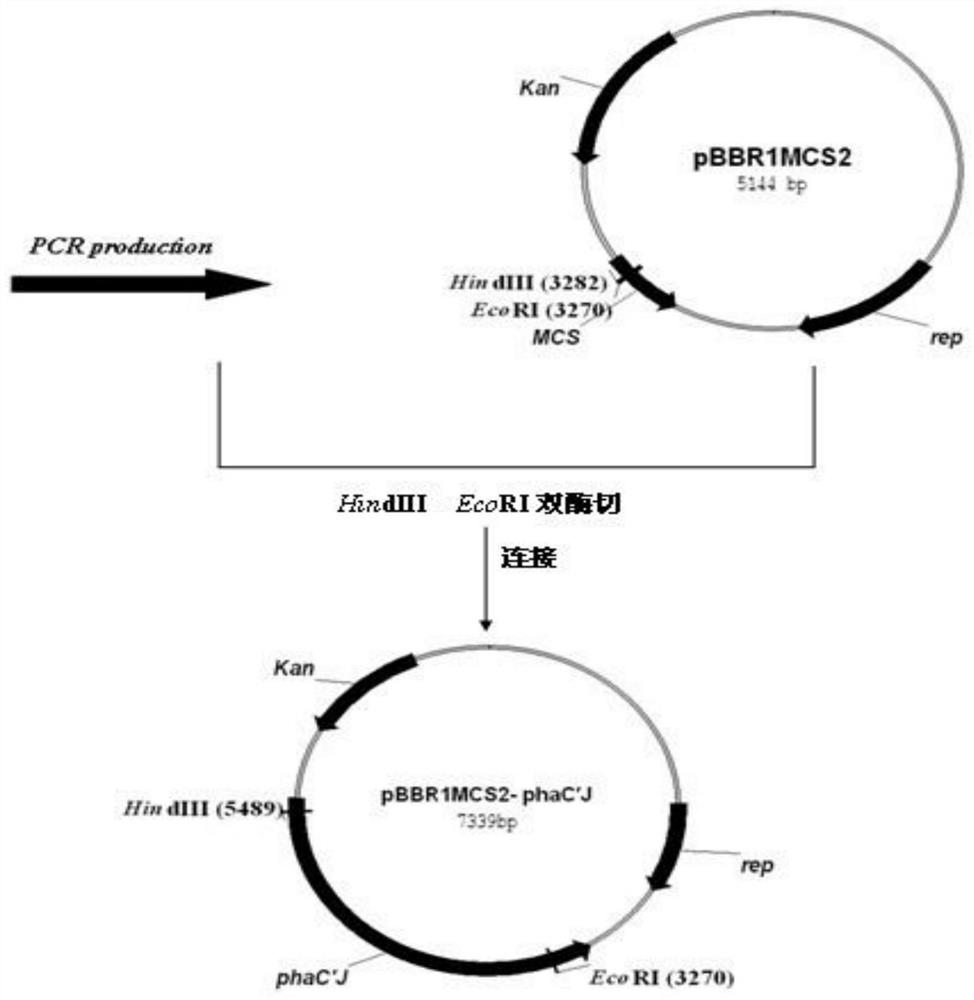
The invention relates to the field of PHA biosynthesis, in particular to a novel PHA (polyhydroxyalkanoate) production method. A specific implementation scheme of the PHA production method comprises the following steps: taking pseudomonas corrugata as an original strain; verifying functional activity of pseudomonas corrugata pBBR1MCS2-phaC2 synthase through expression plasmids of pseudomonas corrugata pBBR1MCS2-phaC1 and pBBR1MCS2-phaC2 by using selected different carbon sources such as fructose, glycerol, lauric acid, sodium gluconate and sodium oleate; and confirming that the PHA can be generated by the recombinant strain by using the sodium oleate as the carbon source according to corresponding results. Therefore, successful construction of the chimeric enzyme in the future is ensured; and moreover, the universality of the substrate of the chimeric PHA synthase can be expanded by the chimeric PHA synthase, thereby increasing the yield of the PHA so as to greatly promote wide application of the PHA.
Owner:LIAONING UNIVERSITY
Microorganism having multiple genes encoding pha synthase and method for producing pha using same
ActiveUS20170198313A1Improve crystallization speedImprove melt workabilityRecombinant DNA-technologyAcyltransferasesMicroorganismBlow molding
A PHA copolymer which is slowly crystallized is improved in crystallization speed to improve the melt workability of the PHA copolymer in working such as injection molding, film molding, blow molding, fiber spinning, extrusion foaming or bead foaming, thereby improving the resultant articles in productivity. A method for the improvement is a method for producing a PHA mixture, including the step of culturing a microorganism having both of a gene encoding a PHA synthase that synthesizes a copolymer PHA (A) and that is derived from the genus Aeromonas, and a gene encoding a PHA synthase that synthesizes a PHA (B) different in melting point from the copolymer PHA (A) by 10° C. or more to produce, in a cell of the microorganism, two or more PHAs different in melting point from one another by 10° C. or more simultaneously.
Owner:KANEKA CORP
Method and application for constructing PHA synthase N-terminal deletion mutant
PendingCN113046287AIncrease productionHigh catalytic activityBacteriaMicroorganism based processesEnzyme digestionGenetic engineering
The invention relates to the field of molecular biology, in particular to a method for constructing a PHA synthase N-terminal deletion mutant. The method comprises the following steps that aeromonas hydrophila PHA synthase is cloned by designing a primer; and then a recombinant plasmid with deletion mutation of amino acids from the 2<nd> site to the 28 site at the N terminal is constructed. After confirming that the expression vector is successfully constructed through enzyme digestion sequencing, transforming the expression vector into an eutrophic alcaligenes mutant strain PHB-4, selecting different carbon sources, performing shake flask fermentation to verify the functional activity of PHA synthase, and then the PHA synthase is compared with wild aeromonas hydrophila. According to the method for constructing the PHA synthase N-terminal deletion mutant, the synthase gene with the mutated N end has the function of generating the PHA, and the yield of the PHA is higher than that of wild fungi, so that the yield of the PHA is increased; and a theoretical basis is provided for constructing a chimeric enzyme for producing novel PHA by using a genetic engineering technology in the future, and certain value in promoting wider application of PHA is achieved.
Owner:LIAONING UNIVERSITY
Preparation method of lactate polymers and lactate copolymers using polyhydroxyalkanoate synthase mutants
The present invention relates to diverse polyhydroxyalkanoate synthase (PHA synthase) mutants capable of synthesizing lactate polymers (PLAs) and lactate copolymers (PLA copolymers), and a preparation method of the lactate polymers and copolymers using the PHA synthase mutants. More specifically, the present invention relates to polyhydroxyalkanoate synthase mutants having SEQ. ID. NO: 2, 4, 6 or 8; and a preparation method of PLA polymers and PLA copolymers using the synthase mutants. According to the present invention, polyhydroxyalkanoate synthase mutants having SEQ. ID. NO: 2, 4, 6 or 8 can have PLA polymer and copolymer synthase activity by the variation in amino acid sequence that affects the PLA polymer synthase activity. Further, the use of such synthase mutants makes it possible to prepare PLA polymers and copolymers having different properties from each other.
Owner:LG CHEM LTD +1
Microorganism having multiple genes encoding PHA synthase and method for producing PHA using same
ActiveUS10519473B2Improve crystallization speedIncrease speedRecombinant DNA-technologyAcyltransferasesProduction rateMicroorganism
Owner:KANEKA CORP
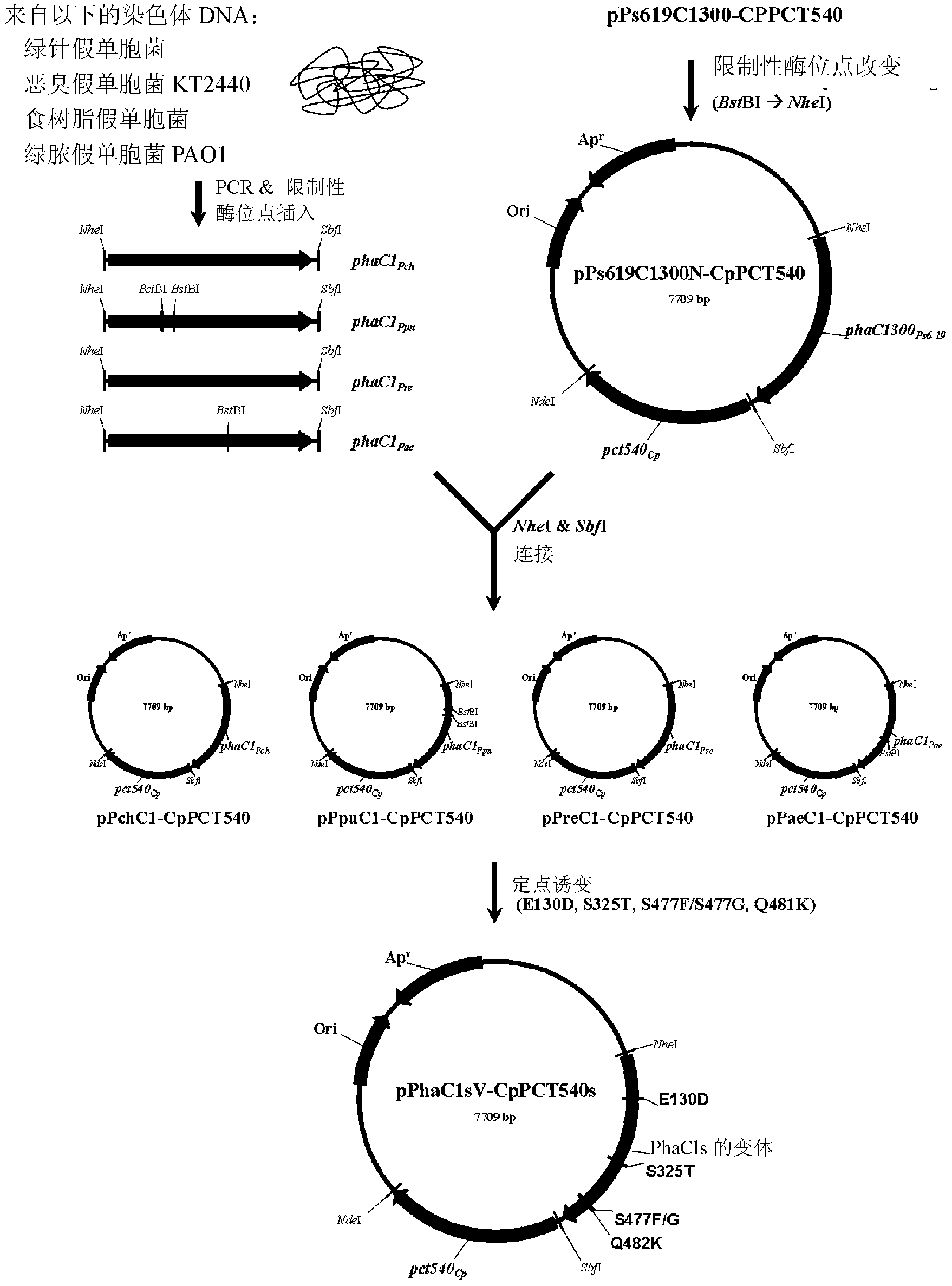
Recombinant bacterium containing novel PHA chimeric enzyme and preparation method and application of recombinant bacterium
PendingCN112831454AIncrease productionSolve problems that consist of a singleBacteriaAntibody mimetics/scaffoldsMonomer compositionPseudomonas hydrophila
The invention relates to a recombinant bacterium containing a novel PHA chimeric enzyme and a preparation method and application of the recombinant bacterium. The method comprises the following steps: by taking a PHA synthase gene phaCRe of oxygen-enriched alcaligenes and aWQ PHA synthase gene phaCAh of aeromonas hydrophila as parents, predicting secondary structures of PhaCRe and PhaCAh by utilizing a database, finding proper binding sites of two enzymes, and constructing the chimeric enzyme PhaCAhRe through SOE PCR. According to the method disclosed by the invention, the constructed chimeric enzyme PhaCAhRe can generate novel PHA by utilizing a proper carbon source, so that the yield of the PHA is increased, and the problem of single composition of a PHA monomer is solved. The invention provides a theoretical basis for constructing a chimeric enzyme for producing novel PHA by using a genetic engineering technology in future, provides a method for changing the monomer composition of PHA, and has certain value in promoting wider application of PHA.
Owner:LIAONING UNIVERSITY
Polyhydroxyalkanoate synthase and gene encoding the same enzyme
A novel polyhydroxyalkanoate (PHA) synthase derived from a microorganism capable of producing a PHA having a novel side-chain structure and a DNA encoding the amino acid sequence for the synthase are provided. Two PHA synthase proteins (SEQ ID NOs: 1 and 3) derived from Pseudomonas cichorii YN2 (FERM BP-7375) and PHA synthase genes encoding these PHA synthases are provided, respectively (SEQ ID NOs: 2 and 4). A recombinant microorganism is endowed with a PHA producing ability.
Owner:CANON KK
Pha-producing genetically engineered microorganisms
InactiveUS20150203878A1Accelerate the accumulation processPrevent slippingBacteriaHydrolasesBiotechnologyMicroorganism
The present invention is directed at genetically engineered form of a naturally PHA producing microorganism, which has an increased number of copies, compared to the wild type microorganism, of at least one gene coding a polyhydroxyalkanoate (PHA) synthase, wherein said increased number of copies provides a balanced overproduction of said PHA synthase, and eventually causing the microorganism to overproduce medium- or long-chain-length PHAs in an amount of at least 1.2 times compared to the wild type after 24 h, wherein the reference condition for assessing the overproduction is modified MM medium containing 15 mM sodium octanoate. The production of PHAs in the microorganism can in addition be favourably influenced by the inactivation of genes encoding for proteins involved in the degradation of PHA, resulting in an even increased production of the microorganism of this compound without a decline in the PHA content over time. The inventive microorganisms are useful in the commercial production of PHAs. The present invention further relates to a method for the production of PHA.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
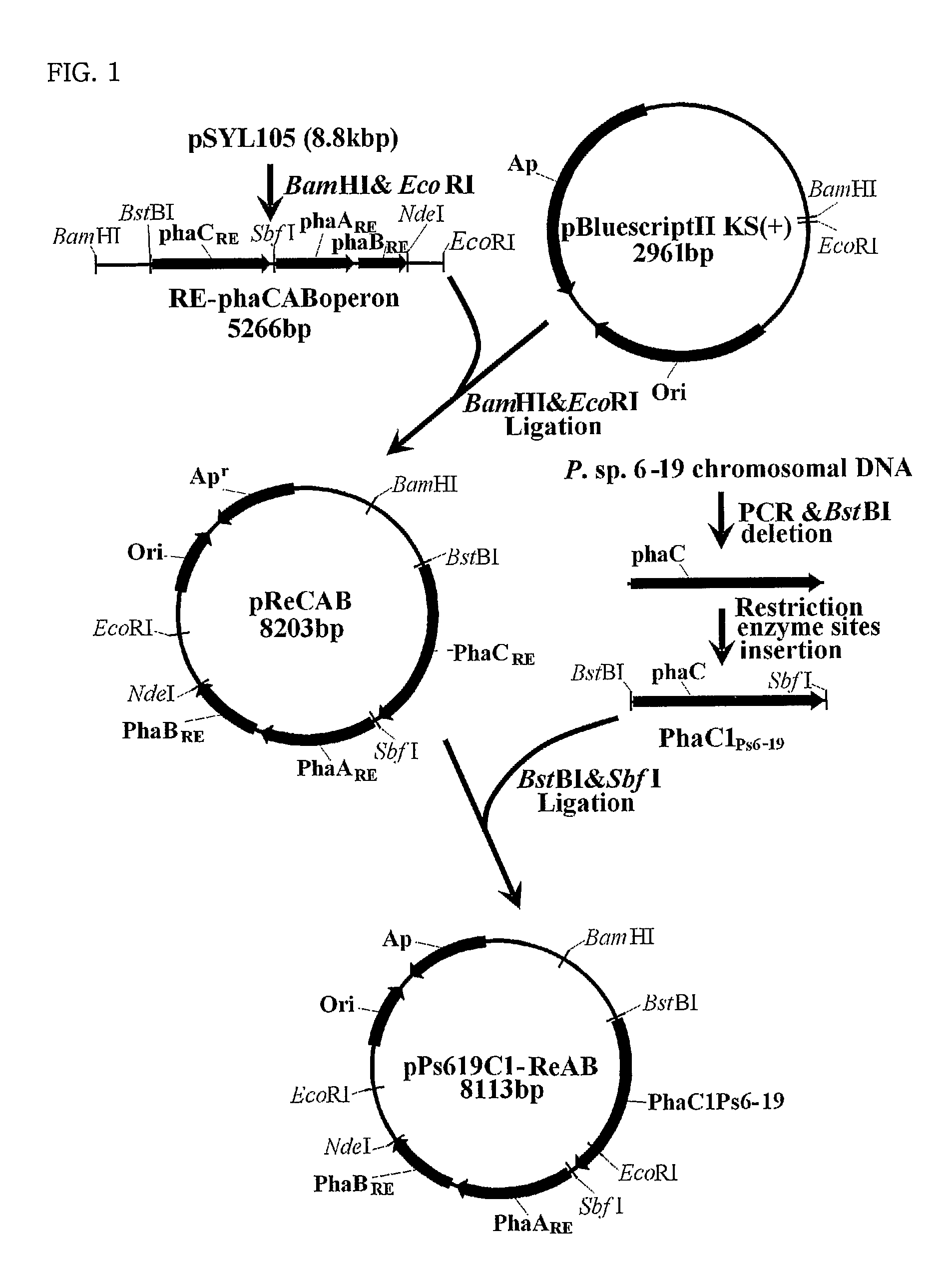
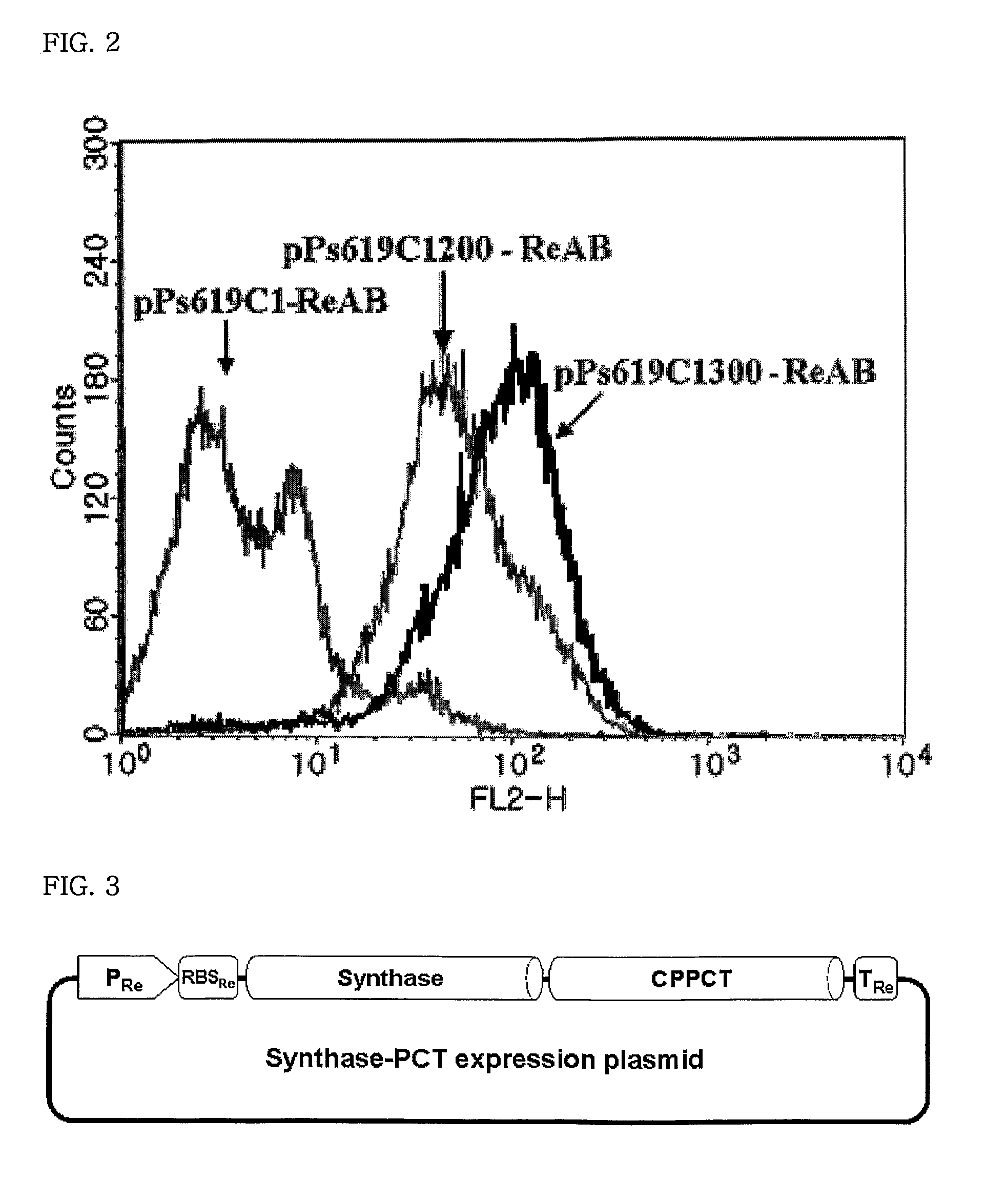
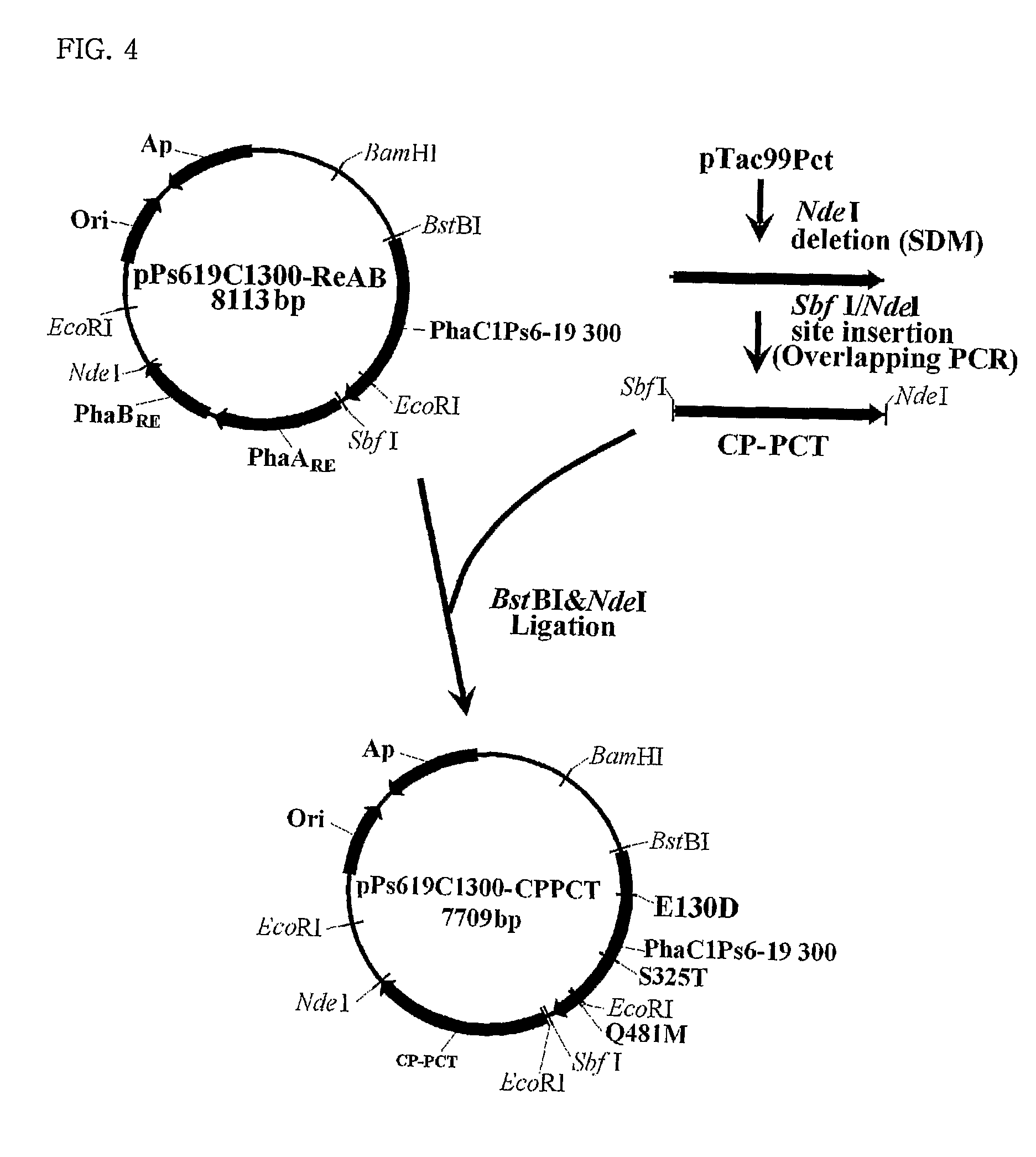
Mutants of PHA synthase from Pseudomonas sp. 6-19 and method for preparing lactate homopolymer or copolymer using the same
ActiveUS8541652B2Easy to useEfficient productionSugar derivativesBacteriaGenus PseudomonasPHA synthase
The present invention relates to polyhydroxyalkanoate synthase (PHA synthase) mutant originated from Pseudomonas sp. 6-19 (KCTC 11027BP) which can prepare lactate polymer and / or copolymer by using lactyl-CoA as a substrate. The present invention relates to a method for preparing lactate polymer and / or copolymer with the synthase mutant. The polyhydroxyalkanoate synthase mutants of the present invention originated from Pseudomonas sp. 6-19 can efficiently prepare lactate polymer and / or copolymer by using as a substrate lactyl-CoA which is difficult to be used as a substrate by conventional polyhydroxyalkanoate synthase.
Owner:LG CHEM LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com