Photo-electricity function material-perylene bisimide type derivative and preparation method thereof
A photoelectric functional material, perylene imide technology, applied in photovoltaic power generation, circuits, electrical components, etc., can solve the problems of low photoelectric conversion efficiency, low solar light absorption efficiency, hindering industrialization, etc., and achieves strong practicability and expansion. The effect of absorption range and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
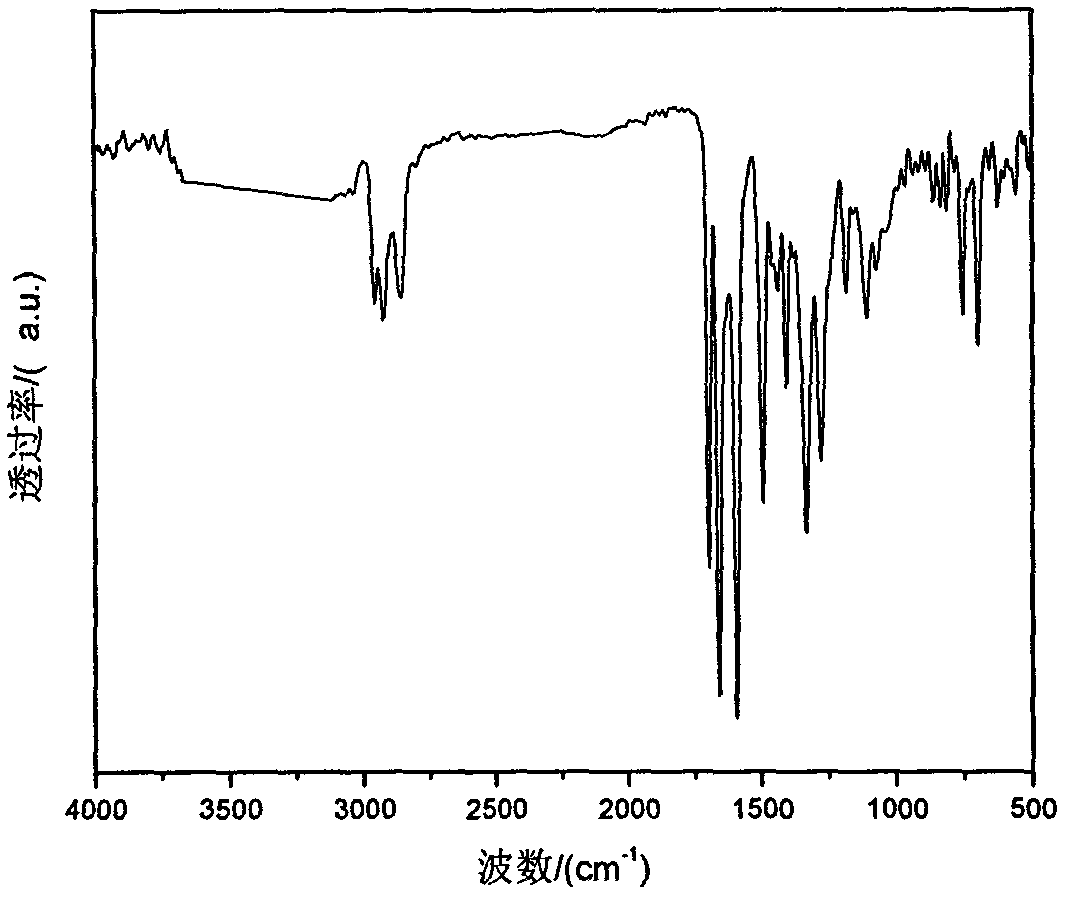
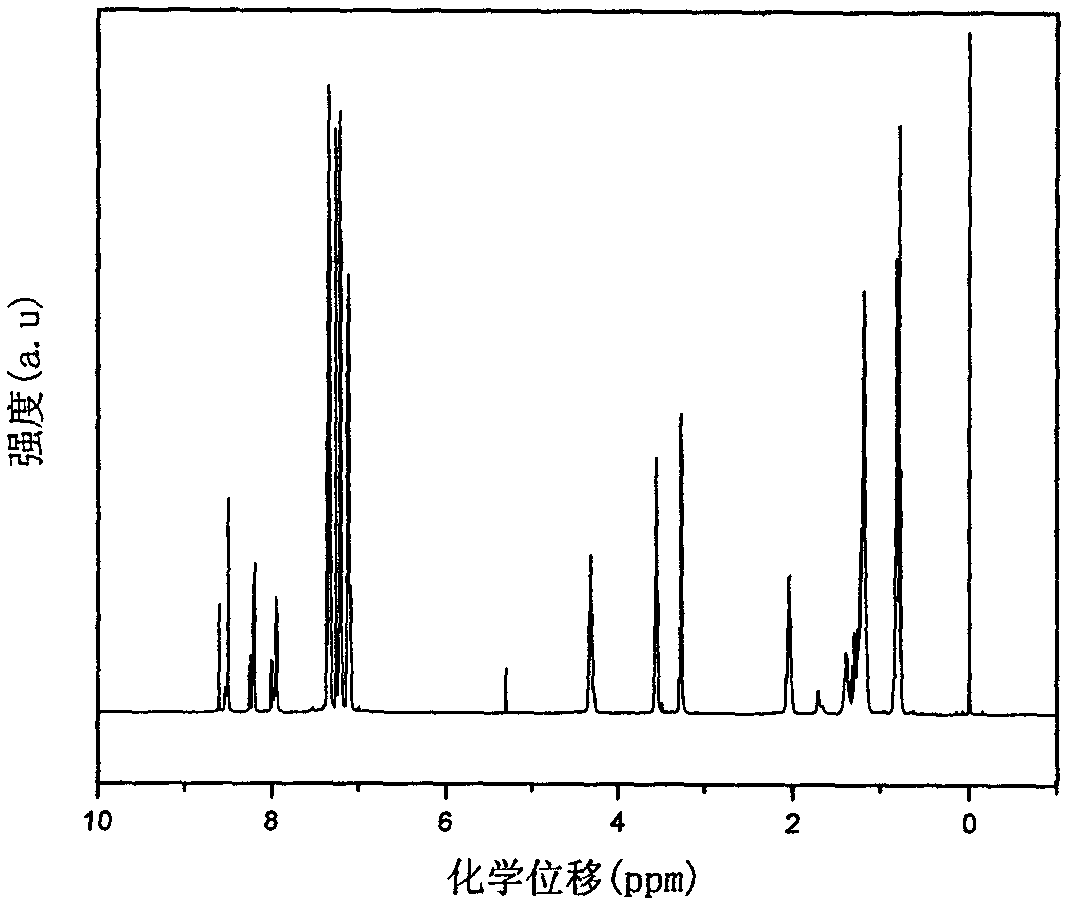
Image
Examples
Embodiment 1
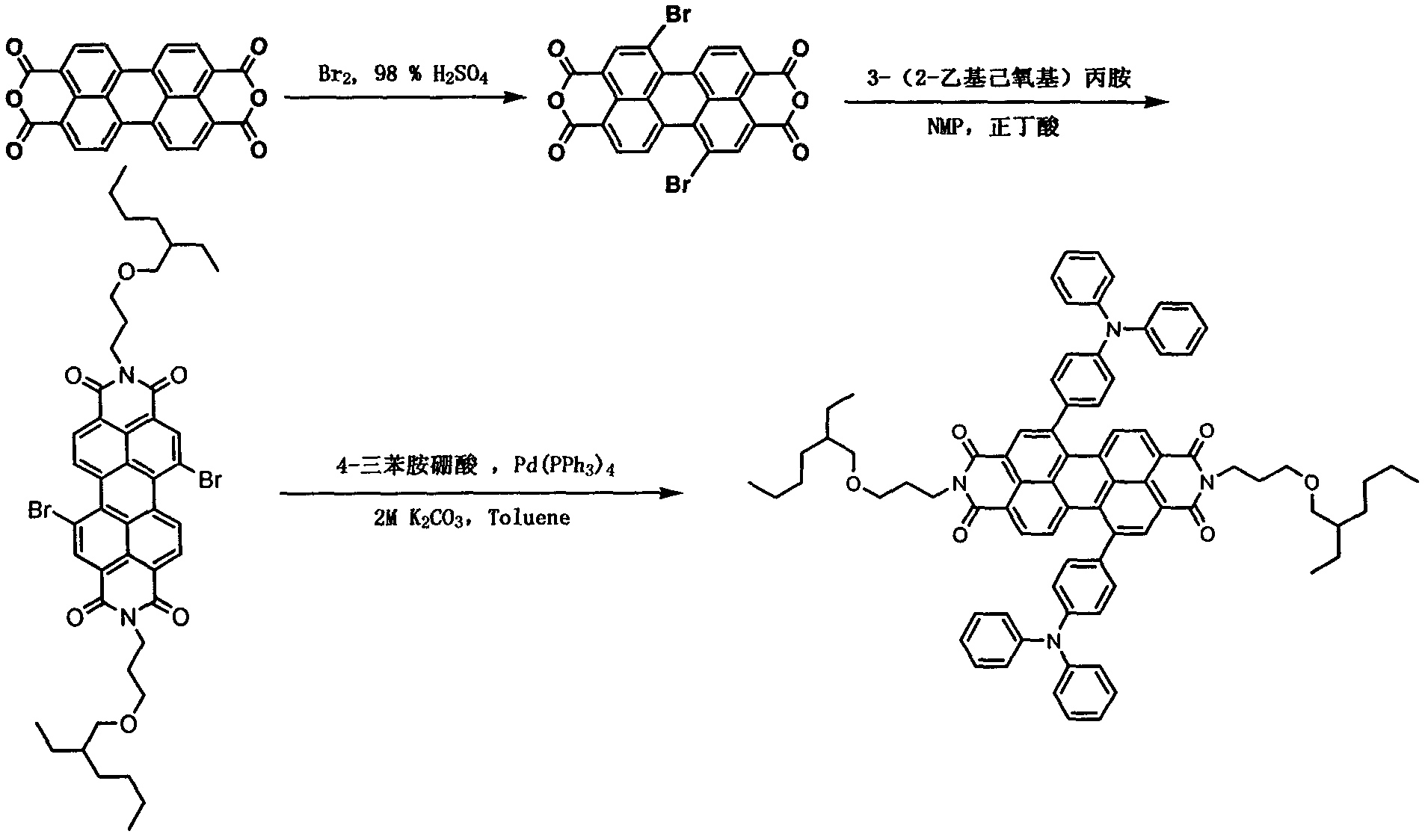
[0018] (1) Weigh the raw material perylenetetracarboxylic acid (10.00g, 25.51mmol) into a 500mL three-neck flask, then add 150mL of concentrated sulfuric acid, and stir at 55°C for 24h. Iodine (0.24 g, 0.95 mmol) was weighed and added to the reaction mixture, and reacted at 55° C. for 5 h. Liquid bromine (2.83 mL, 55.22 mmol) was measured and added dropwise slowly with a constant pressure funnel, and then the reactant was stirred at 85° C. for 24 h. After the reaction, blow off the remaining Br in the reaction device with nitrogen 2 , 50 mL of water was slowly dropped into the cooled reaction mixture, and the precipitate was collected by filtration. The precipitate was washed with 100 mL of 86% concentrated sulfuric acid, filtered, followed by the same operation as above with 150 mL of water, and dried to obtain the product 1,7-dibromo-perylene tetraanhydride with a yield of 85%.
[0019] (2) Weigh compound 1,7-dibromo-perylenetetraanhydride (1.65g, 3.00mmol) into a 100mL th...
Embodiment 2
[0022] (1) Weigh the raw material perylenetetracarboxylic acid (10.00g, 25.51mmol) into a 500mL three-neck flask, then add 150mL of concentrated sulfuric acid, and stir at 55°C for 24h. Iodine (0.24 g, 0.95 mmol) was weighed and added to the reaction mixture, and reacted at 55° C. for 5 h. Liquid bromine (2.83 mL, 55.22 mmol) was measured and added dropwise slowly with a constant pressure funnel, and then the reactant was stirred at 85° C. for 36 h. After the reaction, blow off the remaining Br in the reaction device with nitrogen 2 , 50 mL of water was slowly dropped into the cooled reaction mixture, and the precipitate was collected by filtration. The precipitate was washed with 100 mL of 86% concentrated sulfuric acid, filtered, followed by the same operation as above with 150 mL of water, and dried to obtain the product 1,7-dibromo-perylene tetraanhydride with a yield of 83%.
[0023] (2) Weigh compound 1,7-dibromo-perylenetetraanhydride (1.65g, 3.00mmol) into a 100mL th...
Embodiment 3
[0026](1) Weigh the raw material perylenetetracarboxylic acid (10.00g, 25.51mmol) into a 500mL three-neck flask, then add 150mL of concentrated sulfuric acid, and stir at 55°C for 24h. Iodine (0.24 g, 0.95 mmol) was weighed and added to the reaction mixture, and reacted at 55° C. for 5 h. Liquid bromine (2.83 mL, 55.22 mmol) was measured and added dropwise slowly with a constant pressure funnel, and then the reactant was stirred at 85° C. for 48 h. After the reaction, blow off the remaining Br in the reaction device with nitrogen 2 , 50 mL of water was slowly dropped into the cooled reaction mixture, and the precipitate was collected by filtration. The precipitate was washed with 100 mL of 86% concentrated sulfuric acid, filtered, followed by the same operation as above with 150 mL of water. After drying, the product 1,7-dibromo-perylene tetraanhydride was obtained with a yield of 84%.
[0027] (2) Weigh compound 1,7-dibromo-perylenetetraanhydride (1.65g, 3.00mmol) into a 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap