Thienothiophene quinone-type organic photoelectric material, its preparation method and application
A technology of organic optoelectronic materials and thiophene quinoids, which can be used in light-emitting materials, chemical instruments and methods, circuits, etc., and can solve problems such as low photoelectric conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
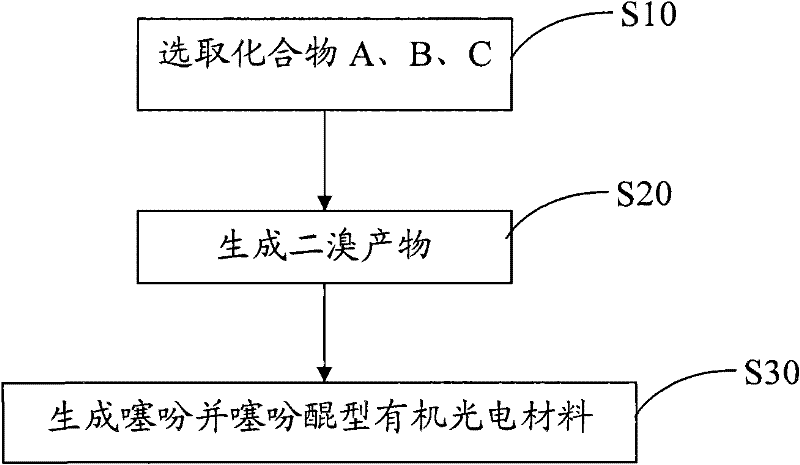
[0032] see figure 2 , the preparation method of the above-mentioned thienothiophene quinone type organic photoelectric material comprises the following steps:
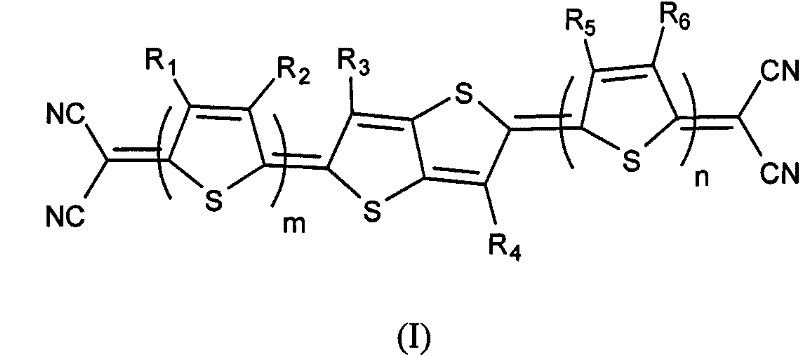
[0033] S10, selecting compounds A, B, and C: that is, selecting compounds A, B, and C represented by the following structural formula,
[0034] Where: R 1 , R 2 , R 3 , R 4 , R 5 , R 6 Choose from H, C 1 -C 20 Alkyl or C 1 -C 20 Alkoxy; m, n are integers of 0-10;
[0035] S20, generation of dibromo product: dissolving compound C and alkyllithium in an organic solvent, then adding trialkyltin chloride, stirring and reacting to generate compound C substituted by alkyltin, and then under the conditions of catalyst and organic solvent , select compound A, B and compound C substituted by alkyltin to carry out Stille coupling reaction, then carry out bromination substitution reaction between Stille coupling reaction product and brominating agent, generate dibrominated product; or carry out bromination of compou...
Embodiment 1
[0078] The thienothiophene quinone type organic semiconductor material of the present embodiment (I 1 ), m=n=0, R 3 , R 4 Both are H, and its structural formula is as follows:
[0079]
[0080] The preparation of the above-mentioned thienothiophene quinone-type organic semiconductor material is described in the above-mentioned case of m=n=0, and the following specific steps are adopted in this embodiment:
[0081] One, prepare the dibromo product 2 of compound C, 5-dibromo-thieno[3,2-b]thiophene, the specific structural formula of the dibromo product in the present embodiment is as follows:
[0082]
[0083] The specific preparation process is as follows: Add thieno[3,2-b]thiophene (unsubstituted compound C) and NBS to tetrahydrofuran at a molar ratio of 1.0:2.0 at -5°C to 30°C, and react for 12 to 48 hours , to obtain the product, namely the dibromo product of the above structural formula. After the reaction was completed, the reaction solution was poured into ice w...
Embodiment 2
[0087] The thienothiophene quinone type organic semiconductor material of the present embodiment (I 2 ), m=n=1, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 Both are H, and its structural formula is as follows:
[0088]
[0089] The preparation steps of the above-mentioned thienothiophene quinone type organic semiconductor material are as follows:
[0090] One, the preparation of 2,5-bis(trimethyltin)thieno[3,2-b]thiophene, its structural formula is as follows:
[0091]
[0092] The preparation process is as follows: add 2.80 g of thieno[3,2-b]thiophene and 80.0 mL of anhydrous THF solution, and add 40.00 mL of n-butyllithium solution (concentration: 2.00 mol / L, at In n-hexane solution), after stirring for 2 hours, add 15.99g of trimethyl tin chloride, continue to stir for 24 hours. After the reaction was completed, the reaction liquid was returned to room temperature, and saturated ammonium chloride aqueous solution was added, extracted with ether, dried over anhydrous mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com