Copolymer containing benzotriazol group as well as preparation method and application of copolymer
A technology of benzotriazole-based copolymers, which is applied in the field of preparation of benzotriazole-based copolymers to achieve stable luminescence performance, good hole transport performance, and reduced manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
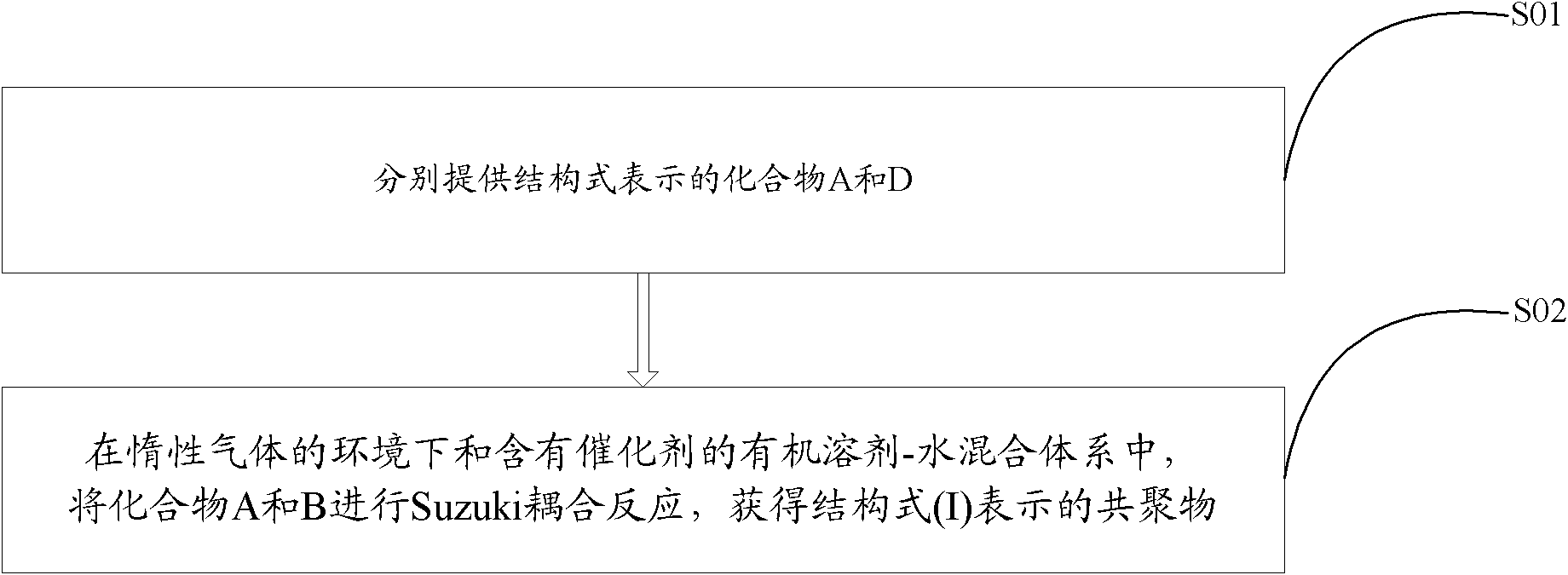
[0033] see figure 2 , the preparation method of above-mentioned benzotriazole-based copolymer comprises the steps:
[0034] S01: Provide compounds A and B represented by the following structural formula, respectively,
[0035] Among them, R is C 1 ~C 32 Alkyl or C 6 ~C 18 Alkoxy monosubstituted phenyl, R' is C 1 ~C 32 the alkyl group;
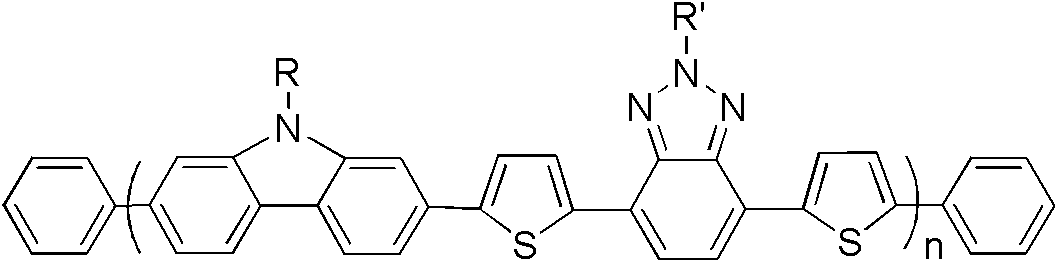
[0036] S02: In an oxygen-free environment, add compounds A and B into an organic solvent containing a catalyst and an alkali solution, and perform a Suzuki coupling reaction at 80-100° C. for 36-60 hours to obtain the compound represented by the following structural formula (I): Benzotriazole based copolymers:
[0037]
[0038] n in the structural formula (I) is an integer of 10-50.
[0039] In step S01, compounds A and B can be directly purchased from the market or prepared by existing synthetic methods. Wherein, it is basically the same as the description of the above-mentioned benzotriazole-based copolymer, R is preferably C ...
Embodiment 1
[0056] The poly{9-(4'-n-octyloxyphenyl)-2,7-carbazole-co-2-n-octyl-4,7-dithienylbenzotriazole} (PCDTBTz1) of this embodiment , its structural formula is as follows:
[0057]
[0058] The preparation steps of the above-mentioned PCDTBTz1 polymer are as follows:
[0059] The preparation of step 1, 2-n-octylbenzotriazole, reaction formula is as follows:
[0060]
[0061] The specific process is as follows: 1,2,3-benzotriazole (5.0g, 42mmol), potassium hydroxide (5.6g, 100mmol) and n-octane bromide (9.5g, 49mmol) were dissolved in 50mL of methanol, heated Reflux for 24h. After cooling to room temperature, the stirring was stopped, and the solvent was removed by rotary evaporation. The remaining substance was poured into 200 mL of chloroform, washed twice with water to separate the organic phase, dried with anhydrous magnesium sulfate, and the solvent was removed. The crude product was separated by silica gel column chromatography using petroleum ether as eluent to obtain ...
Embodiment 2
[0081] The poly{9-(4'-n-octyloxyphenyl)-2,7-carbazole-co-2-n-dodecyl-4,7-dithienylbenzotriazole}PCDTBTz of this embodiment 2. Its structural formula is as follows:
[0082]
[0083] The preparation process is described in detail below.
[0084] The preparation of step 1, 2-n-dodecylbenzotriazole, reaction formula is as follows:
[0085]
[0086] The specific process is as follows: 1,2,3-benzotriazole (5.0g, 42mmol), potassium hydroxide (5.6g, 100mmol) and brominated n-dodecane (9.5g, 49mmol) were dissolved in 50mL of methanol, Heated to reflux for 24h. After cooling to room temperature, the stirring was stopped, and the solvent was removed by rotary evaporation. The remaining substance was poured into 200 mL of chloroform, washed twice with water to separate the organic phase, dried with anhydrous magnesium sulfate, and the solvent was removed. The crude product was separated by silica gel column chromatography with petroleum ether as the eluent to obtain a colorless ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com