Compound containing pyridine group, and organic light-emitting device using compound
An organic light-emitting device, pyridine group technology, applied in the field of organic light-emitting devices, can solve problems such as restricting the industrialization of light-emitting devices, and achieve the effects of excellent hole transport performance, good thermal stability, and low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
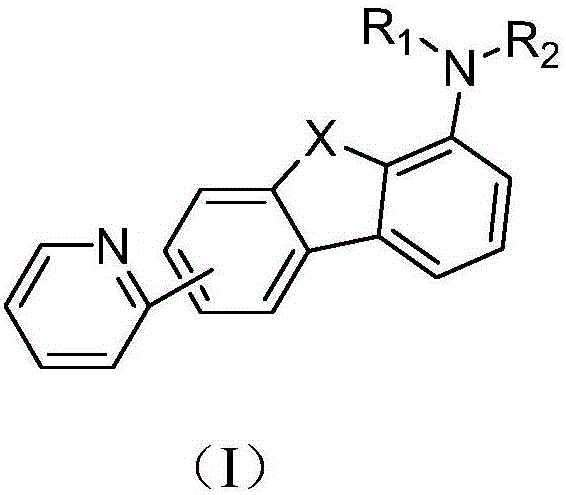
[0040] The preparation method of the pyridine group-containing compound of the present invention comprises reacting the compound represented by the formula (A) and the compound represented by the formula (B) to obtain the pyridine group-containing compound represented by the formula (I).
[0041]
[0042] The present invention has no special requirements on the conditions of the above reactions, and conventional conditions of this type of reactions well known to those skilled in the art will suffice. The present invention has no special limitation on the sources of the raw materials used in the above reactions, which can be commercially available products or prepared by methods well known to those skilled in the art. where the R 1 , R 2 , The choice of X is the same as that described above, and will not be repeated here.
[0043] The present invention also provides an organic light-emitting device, including the compound containing a pyridine group. The organic light-emi...
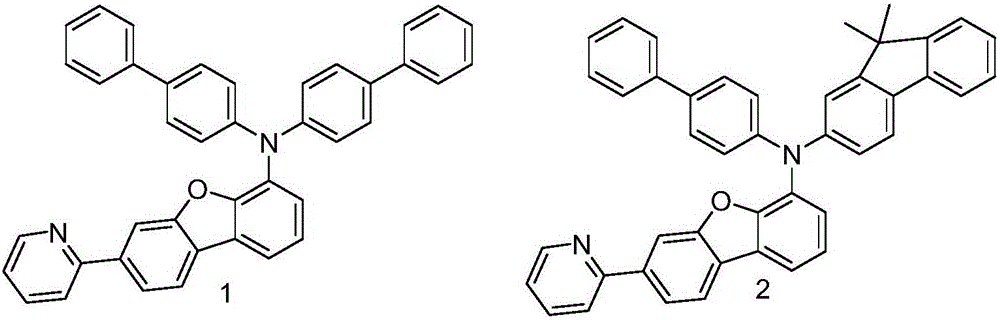
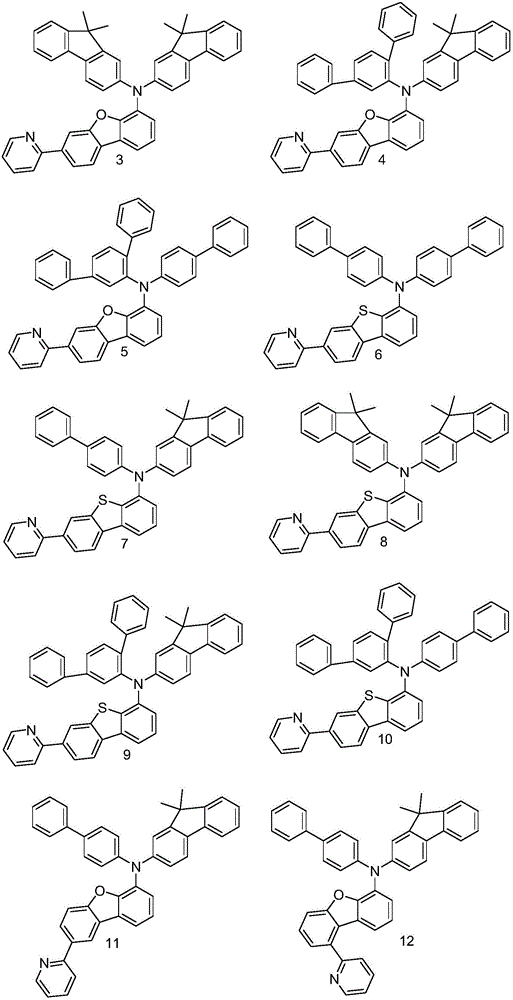
Embodiment 1
[0045] Embodiment 1: the synthesis of intermediate A1
[0046]
[0047]Add 0.1mol p-aminobiphenyl, 0.1mol 3-bromo-N-phenylcarbazole and 600mL tetrahydrofuran and 100ml water into the reaction flask, and add 0.2mol potassium carbonate to it, and add 0.5g tetrakis(triphenyl Base) phosphine palladium, under the protection of nitrogen at room temperature for 12 hours; then, add distilled water to the reaction solution to terminate the reaction, and extract with ethanol / dichloromethane, wash with salt water, separate the organic layer, and remove the organic solvent by rotary evaporation, and the crude product is passed through the column , and then purified by recrystallization with dichloromethane and ethanol to obtain 30.4 g of intermediate A1 with a yield of 84%.
[0048] The following intermediate compounds were obtained in a similar manner:
[0049]
[0050]
Embodiment 2
[0051] Embodiment 2: the synthesis of intermediate B1-1
[0052]
[0053] Dissolve 16.9g (1) in 180ml of glacial acetic acid, slowly add dropwise a mixture of 15ml of liquid bromine and 110ml of glacial acetic acid, and the addition will be completed in about 5 hours. A large amount of solid precipitated out. LC tracking until the disappearance of reactants, stop the reaction. Ice water was added, and 29.5 g of yellow solid powder (2) was obtained by suction filtration, with a yield of 91%.
[0054] With 32.4g yellow solid powder (2), and 133.8g NH 4 Dissolve Cl in 500ml of methanol and 500ml of water, stir at room temperature for 1 hour, add 56g of activated Fe powder, stir the mixture overnight, and filter to remove excess Fe powder. Extract the aqueous phase with ethyl acetate, anhydrous Na 2 SO 4 After drying and concentrating, 23.52 g of intermediate (3) was obtained with a yield of 80%.
[0055] Add 29.5g of intermediate (3) to 300ml of absolute ethanol, then dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com