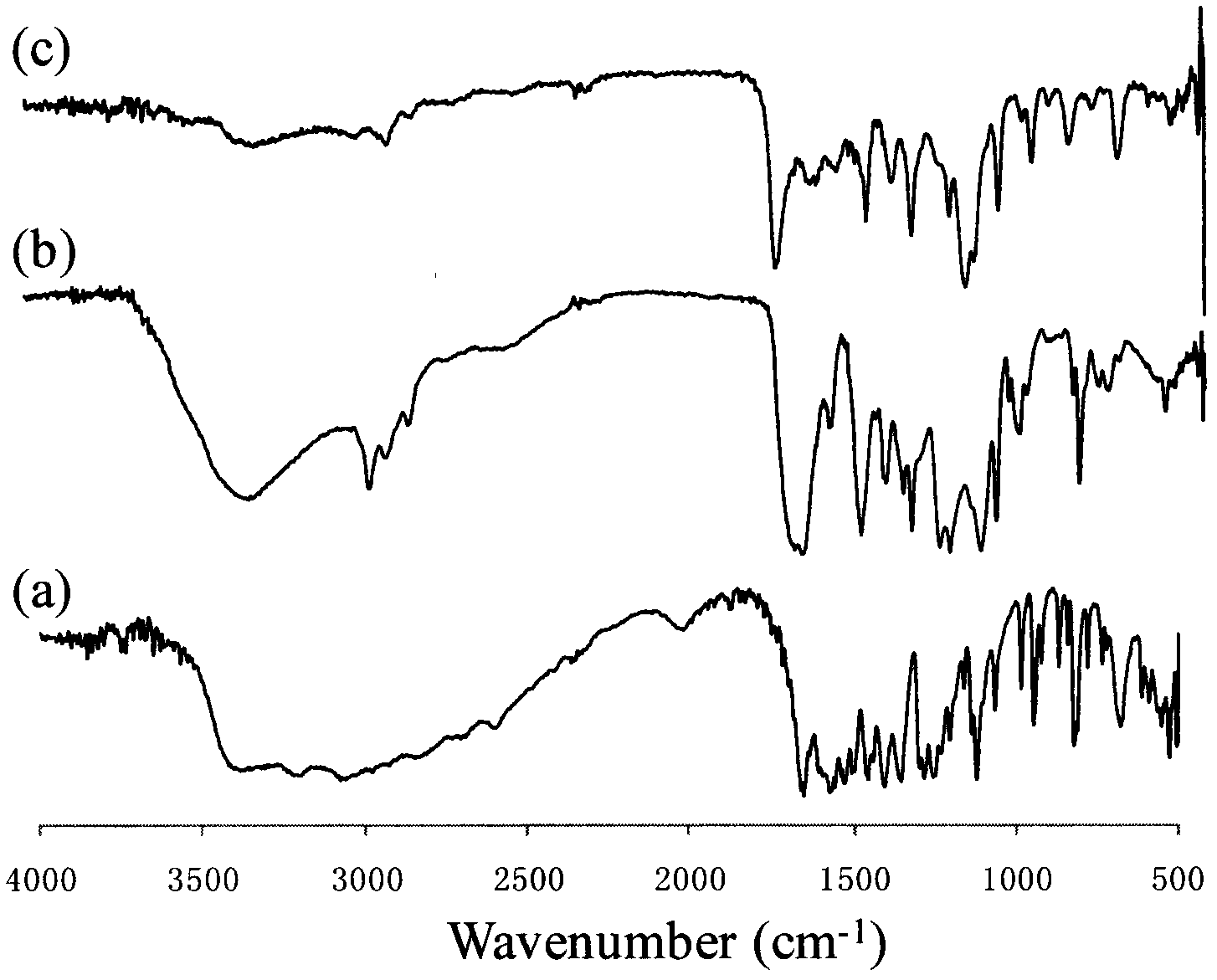
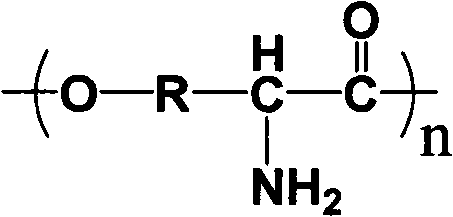
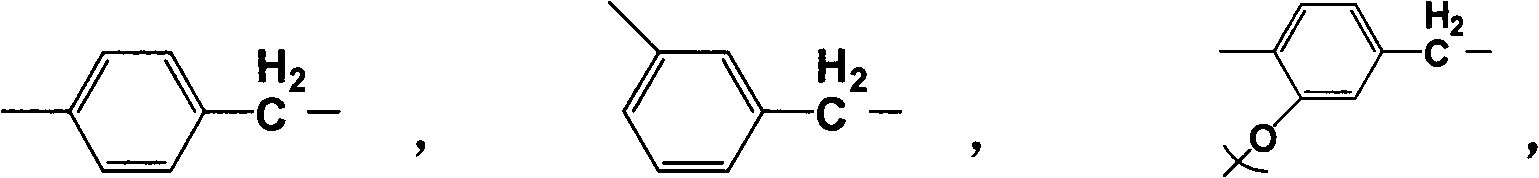
Aromatic amino acid polymer
A polymer, amino acid technology, applied in the field of aromatic amino acid polymers, can solve the problems of preparation, structure and performance, and achieve the effects of wide application value, good degradability, and excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Add 5.916 g (30 mmol) of L-dopa to NaHCO under stirring in an ice bath 3 (7.5wt%) in the aqueous solution; Di-tert-butyl dicarbonate ((Boc) 2 O) 7.857g (36mmol) was dissolved in 50ml 1,4-dioxane, dropped into the reaction system, reacted at 5°C for 30min, then raised the temperature to 25°C under stirring, and reacted for 19.5h; the whole reaction process was carried out under nitrogen and Carry out under dark conditions. After the reaction, adjust the pH of the solution to 2-3 with 1M HCl, then extract with ethyl acetate (100ml×3); add an appropriate amount of anhydrous MgSO 4 After drying, a light yellow oil was obtained, which was L-dopa protected by tert-butoxycarbonyl.
[0020] The second step: 2.98g (10mmol) of L-dopa protected by tert-butoxycarbonyl group, 0.009g (0.1mmol) of sodium acetate, and 15ml of acetic anhydride were added to a 100ml reactor, and the temperature was raised to 140°C. After 1 hour of reaction, the temperature was raised again To ...
Embodiment 2
[0025] Add 5.430g (30mmol) of L-tyrosine to NaHCO 3 (7.5wt%) in the aqueous solution; Di-tert-butyl dicarbonate ((Boc) 2 O) 7.857g (36mmol) was dissolved in 50ml 1,4-dioxane, and other steps were carried out as described in Example 1. The molecular formula of the obtained homopolymer is:
[0026]
[0027] Expressed as PTyr.
Embodiment 3
[0029] Add 5.430 g (30 mmol) of 2-hydroxyphenylalanine to NaHCO 3 (7.5wt%) in the aqueous solution; Di-tert-butyl dicarbonate ((Boc) 2 O) 7.857g (36mmol) was dissolved in 50ml 1,4-dioxane, and other steps were carried out as described in Example 1. The molecular formula of the obtained homopolymer is:
[0030]
[0031] Expressed as P2HPA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com