Butylphthalide controlled release preparation and preparation method thereof
A technology of controlled-release preparation and butylphthalide, which is applied in the field of butylphthalide preparations, can solve the problems of large amount of hydrophilic gel skeleton material, large amount of cyclodextrin, unable to meet the requirements of sustained-release tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
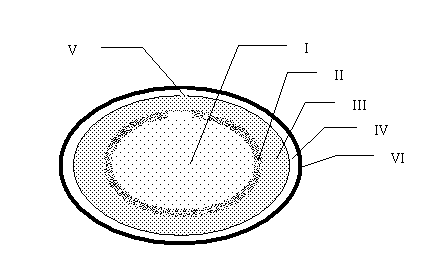
[0055] Embodiment 1, The liquid medicine contains an emulsifier, but the middle layer does not contain an emulsifier.
[0056] .
[0057] Preparation Process:
[0058] ① Preparation of gelatin solution: take gelatin and add appropriate amount of water to make it absorb water and swell. In addition, heat glycerin, ethylparaben and the remaining water in a colloidal sol pot to 70~80°C, mix well, add expanded gelatin, stir, melt, keep warm for 1~2 hours, let stand to make the foam float up, scrape off The floating foam is filtered with a clean white cloth and kept warm for later use. The viscosity of the formulated glue is generally 2.8~3.2 degrees;
[0059] ②Preparation of liquid medicine oil: weigh the components and stir well to get it;
[0060] ③ Pressing soft capsules: Put the prepared gelatin liquid and butylphthalide into the automatic rotary encapsulating machine, control the temperature at 40~50°C, and press out soft capsules;
[0061] ④ Preparation of the middl...
Embodiment 2
[0068] Embodiment 2, Both the liquid medicine and the intermediate layer contain emulsifiers.
[0069] Preparation process steps: ①~③, ⑤~⑩ are the same as in Example 1; ④ Preparation of intermediate layer solution: Dissolve mannitol, ethoxylated polyoxyethylene glyceride and povidone in ethanol.
Embodiment 3
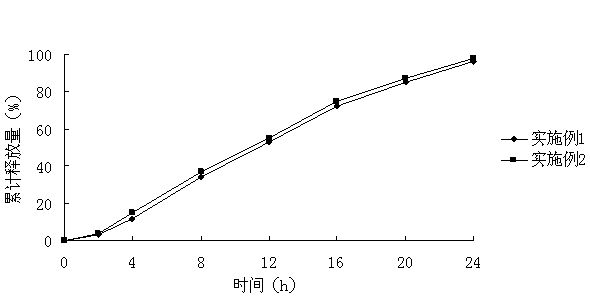
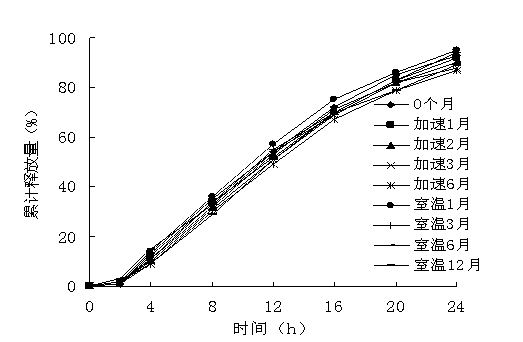
[0070] Embodiment 3, According to the second method of dissolution determination (paddle method) of appendix XC of the Chinese Pharmacopoeia 2010 edition, with pH6.8 phosphate buffer as the dissolution medium, measure Examples 1 and 2, and draw the release curve, the results are shown in the appendix figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com