Method for detecting infectious haematopoietic necrosis virus based on liquid-phase chip
A hematopoietic organ necrosis and infectivity technology, applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc. Polluted environment, short time required, good specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the design of specific primer pair and specific probe
[0038] A specific primer pair and a specific probe for amplifying infectious hematopoietic organ necrosis virus are designed through a large number of sequence alignments and amplification effect comparisons.
[0039] The specific primer pair is as follows (the target sequence is 126bp):
[0040] F1 (sequence 1 of the sequence listing): 5'-ACGGAGTATCGTCCCAGTA-3';
[0041] R1 (SEQ ID NO: 2 of the Sequence Listing): 5'-GAGGCTCAATGCCTTTCT-3'.
[0042] The nucleotide sequence of the specific probe (sequence 3 in the sequence listing) is as follows:
[0043] 5'-TCCTCCGACTTGACTCACCGC-3'.
Embodiment 2
[0044] Example 2, application of specific primer pairs to aid in the identification of infectious hematopoietic organ necrosis virus
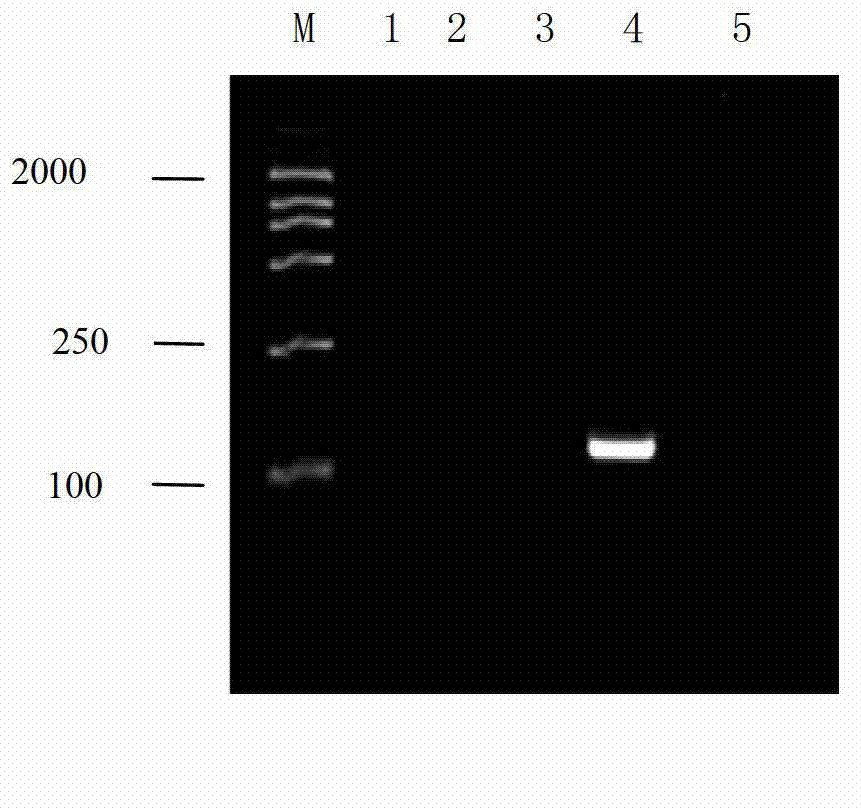
[0045] Infectious hematopoietic necrosis virus, viral hemorrhagic sepsis virus, carp spring viremia virus, infectious pancreatic necrosis virus and viral neuronecrosis virus were used as the viruses to be tested for the following experiments:
[0046] 1. Use the RNA extraction kit to extract the total RNA of the virus to be tested.
[0047] 2. Using the total RNA obtained in step 1 as a template, using a primer pair composed of F1 and R1, and using an RT-PCR kit, perform RT-PCR amplification on a gradient PCR amplification instrument to obtain RT-PCR amplification products.
[0048] RT-PCR amplification reaction system: In a 0.2mL PCR reaction tube, add 10×RT-PCR buffer 2.5μL, dNTP (2.5mmol / L each) 2.5μL, 10pmol / μL F10.5μL, 10pmol / μL R10 .5μL, Inhibiter0.5μL, AMV XL0.5μL, AMV Taq (5U / μL)0.5μL, 25mmol / L MgCl 2 5.0 μL, total RNA 3.0 μL (about 3...
Embodiment 3
[0052] Example 3, application of primer probe composition to assist in the identification of infectious hematopoietic necrosis virus
[0053] 1. Preparation of primers and probes
[0054] Prepare primers and probes as follows:
[0055] F2: 5'-biotin-ACGGAGTATCGTCCCAGTA-3';
[0056] R2: 5'-biotin-GAGGCTCAATGCCTTTCT-3'.
[0057] Probe T1: 5'-NH 2 -TCCTCCGACTTGACTCACCGC-3'.
[0058] F2 is biotinylated at the 5' end of F1, and R2 is biotinylated at the 5' end of R1. Probe T1 is obtained by amination modification at the 5' end of the single-stranded DNA fragment shown in Sequence 3 of the sequence listing.
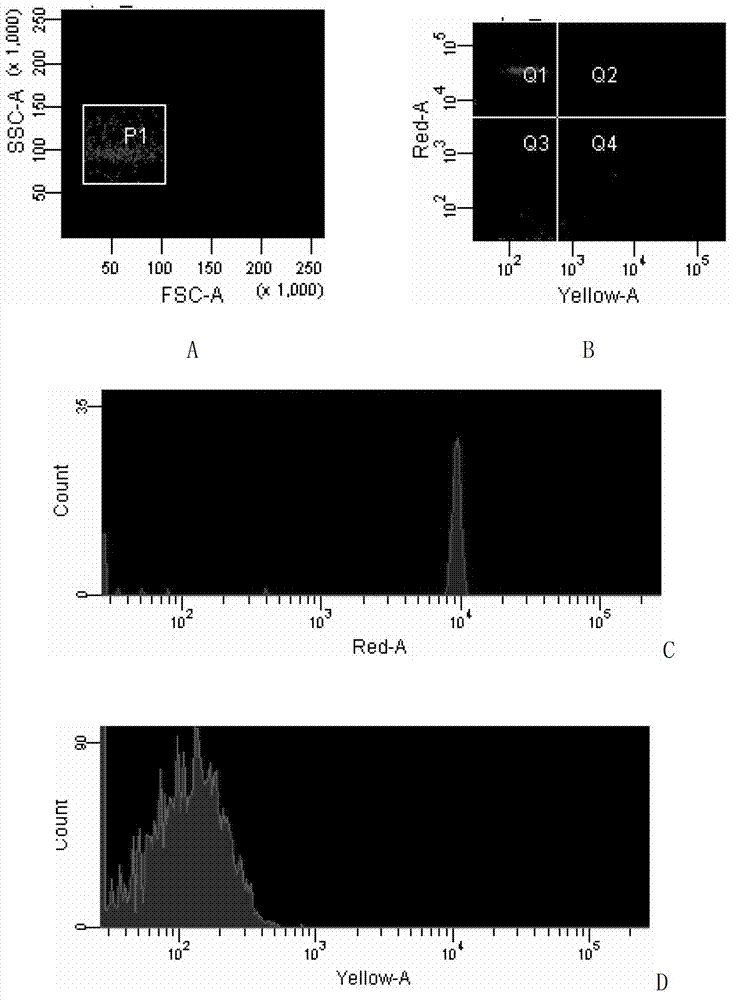
[0059] 2. Establishment of liquid phase chip detection system
[0060] 1. Use the RNA extraction kit to extract the total RNA of infectious hematopoietic organ necrosis virus.
[0061] 2. Using the total RNA obtained in step 1 as a template, using a primer pair composed of F2 and R2, and using an RT-PCR kit, perform RT-PCR amplification on a gradient PCR amplification in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com