Asarin compound and freeze-dried powder injection thereof
A technology of freeze-dried powder injection and asarone, applied in freeze-dried delivery, drug combination, organic chemistry, etc., to achieve ideal stability, ideal curative effect, simple and feasible preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
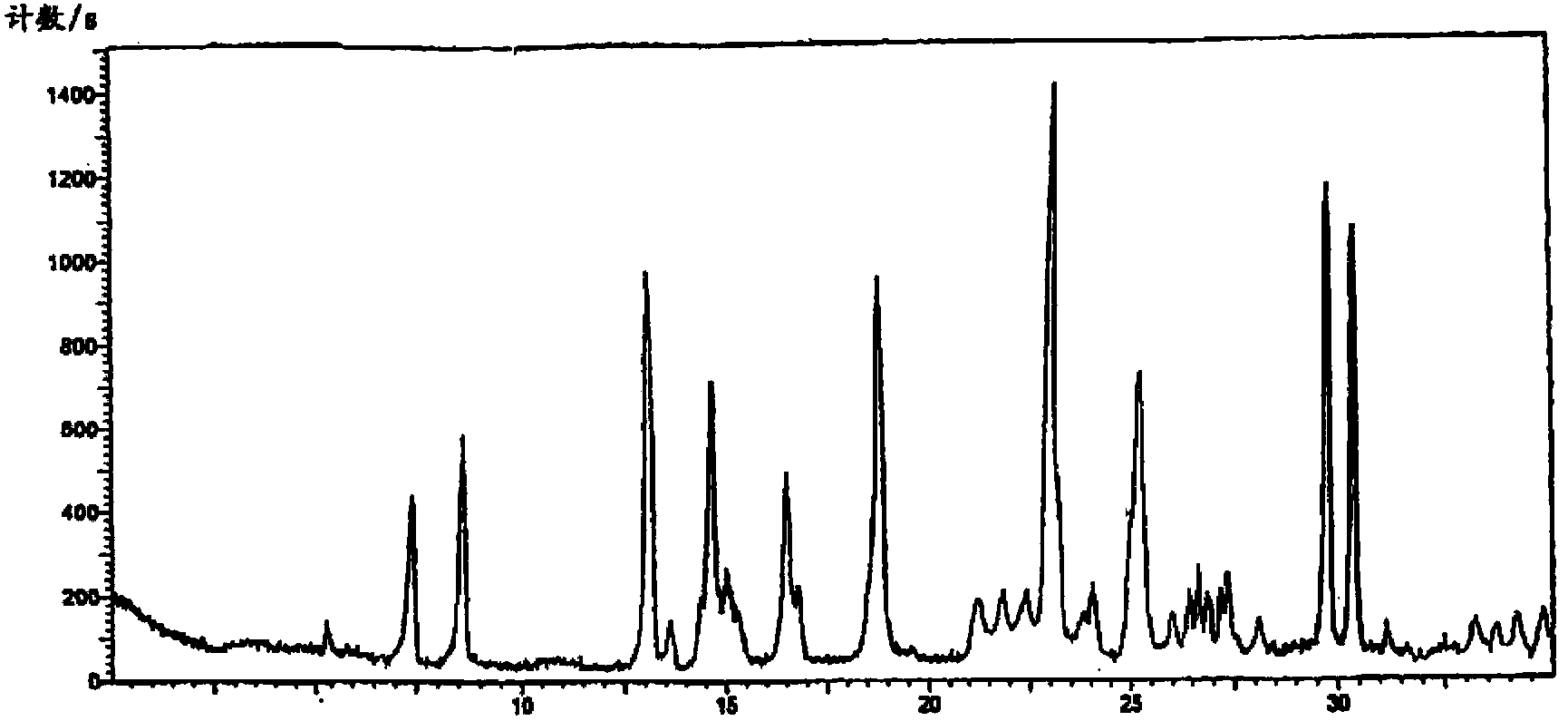
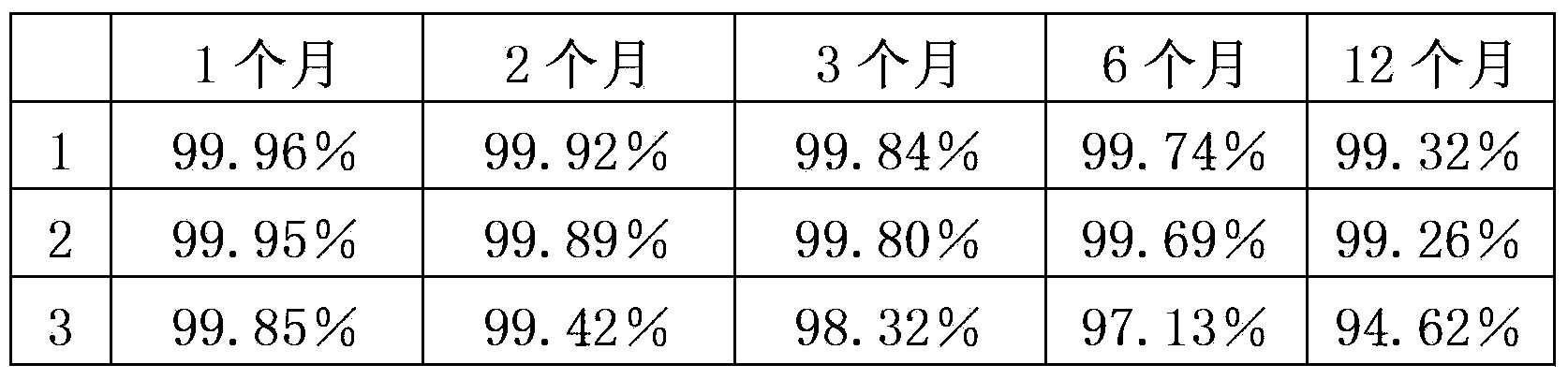
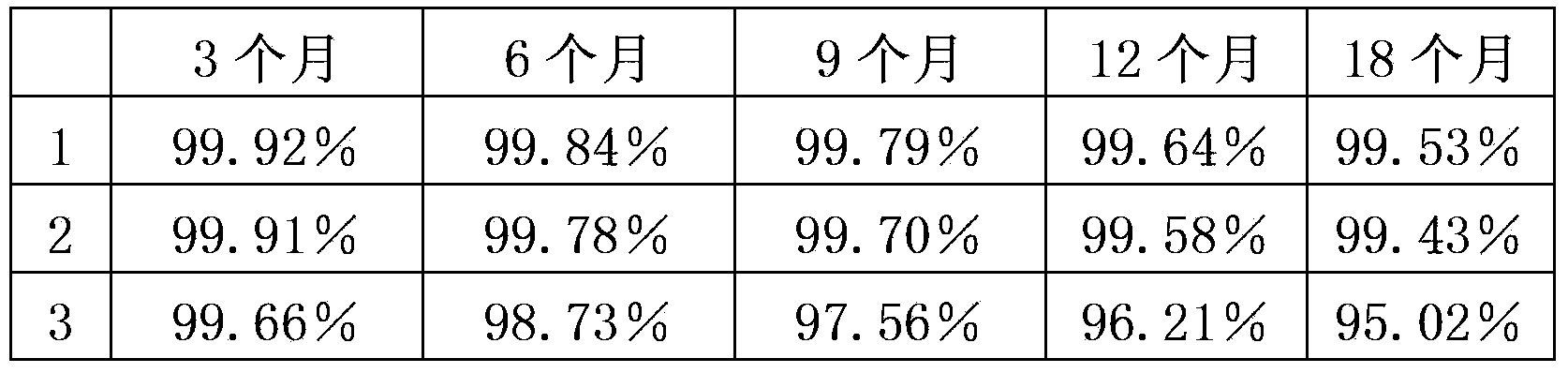
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 asarone compound
[0048] (1) Weigh 100g of asarone crude product, dissolve 96g of it in an organic solvent whose volume is equivalent to 6 times the weight of asarone crude product, and the organic solvent is acetonitrile / In ether / ethanol, stir evenly at a stirring speed of 15rmp to obtain solution A;
[0049] (2) Fully dissolve the remaining 4g of Asarone crude product in 0.05mol / L ethanol aqueous solution at 42°C with a stirring speed of 25rmp to form solution B;
[0050] (3) Within 2.5 hours, add solution B dropwise to solution A at a constant speed, while controlling the temperature of solution A at 22°C, stirring at a low speed of 8rmp during the feeding process;
[0051] (4) After the feeding is completed, the temperature of the solution is lowered to 2°C, placed in an ultrasonic field with a power of 0.2KW to grow crystals for 8 hours, filtered, the filter cake is washed with water, and dried at a low temperature below 40°C to o...
Embodiment 2
[0053] The preparation of embodiment 2 Asarone compounds
[0054] (1) Weigh 100g of asarone crude product, dissolve 95g of it in an organic solvent whose volume is equivalent to 5 times the weight of asarone crude product, and the organic solvent is acetonitrile / In ether / ethanol, stir evenly, the stirring speed is 5rmp, obtain solution A;
[0055] (2) Fully dissolve the remaining 5g of Asarone crude product in 0.075mol / L ethanol aqueous solution at 40°C with a stirring speed of 30rmp to form solution B;
[0056] (3) Within 2 hours, add solution B dropwise to solution A at a constant speed, while controlling the temperature of solution A at 20°C, stirring at a low speed of 5rmp during feeding;
[0057] (4) After the feeding is completed, the temperature of the solution is lowered to 0°C, placed in an ultrasonic field with a power of 0.1KW to grow crystals for 10 hours, filtered, the filter cake is washed with water, and dried at a low temperature below 40°C to obtain the asar...
Embodiment 3
[0059] The preparation of embodiment 3 asarone compounds
[0060] (1) Weigh 100g of asarone crude product, dissolve 98g of it in an organic solvent whose volume is equivalent to 8 times the weight of asarone crude product, and the organic solvent is acetonitrile / In ether / ethanol, stir evenly, the stirring speed is 25rmp, obtain solution A;
[0061] (2) Fully dissolve the remaining 2g of Asarone crude product in 0.025mol / L ethanol aqueous solution at 45°C with a stirring speed of 20rmp to form solution B;
[0062] (3) Within 2-3 hours, add solution B to solution A dropwise at a constant speed, while controlling the temperature of solution A at 25°C, stirring at a low speed of 10rmp during the feeding process;
[0063] (4) After the feeding is completed, the temperature of the solution is lowered to 5°C, placed in an ultrasonic field with a power of 0.3KW to grow crystals for 6 hours, filtered, the filter cake is washed with water, and dried at a low temperature below 40°C to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com