Application of hydroxyl-containing crosslinked polymer guanidinated product in gene transfer
A cross-linked polymer, guanidine-based technology, used in gene therapy, medical preparations with non-active ingredients, genetic material components, etc., to achieve the effects of flexible spatial structure, high reactivity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of the PEI derivative containing hydroxyl
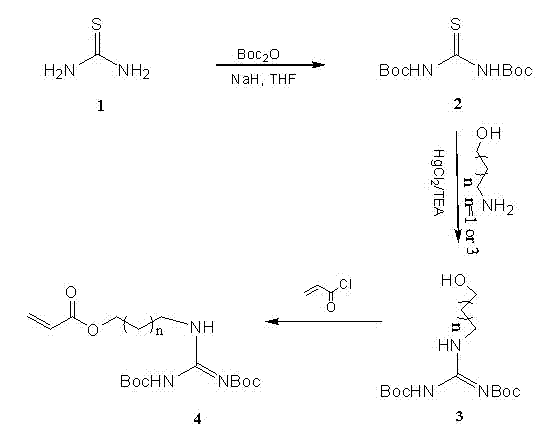
[0050] 0.5 grams of diethylenetriamine (which can be regarded as PEI with a molecular weight of 103) is dissolved in 5ml of dichloromethane and placed in a 50ml reaction bottle. A certain amount of crosslinking agent L1, namely N',N'-tetracyclic Oxypropyl-4,4'-diaminodiphenylmethane (can also be one or two of L2-L5, quantified according to the molar ratio of the reaction) dissolved in 5ml of dichloromethane at 30-35 ° C, Add it dropwise into the above reaction bottle under magnetic stirring, and then continue to react for 22 hours. The volume of the reaction solution is reduced by more than half by rotary evaporation, and then dialyzed for more than 72 hours, and the hydroxyl-containing PEI derivative product is obtained after freeze-drying.
Embodiment 2
[0051] Embodiment 2: the synthesis of the PEI derivative containing hydroxyl
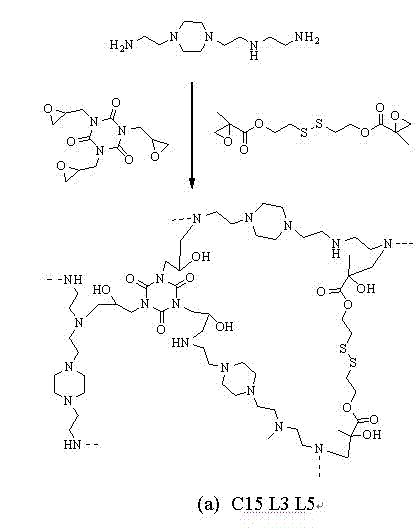
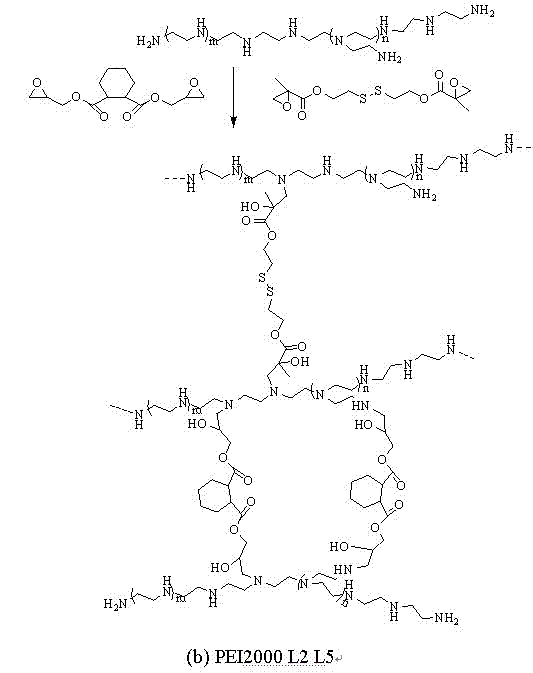
[0052] 0.5 grams of PEI (can be linear or branched, molecular weight 600) dissolved in 5ml of dichloromethane and placed in a 50ml reaction bottle, a certain amount of cross-linking agent L5 (can also be one of L1-L4 or Two kinds, quantified according to the molar ratio of the reaction) dissolved in 5ml of dichloromethane at 20-25°C, added dropwise to the above reaction flask under magnetic stirring, and then continued to react for 18 hours, and the volume of the reaction solution was reduced by rotary evaporation More than half of it was then dialyzed for more than 72 hours, and the hydroxyl-containing PEI derivative product was obtained after freeze-drying. Various hydroxyl-containing PEI derivatives (raw materials are shown in Table 1; Table 2), various reaction combinations and the resulting The polymer structure is schematically shown in Figure 1.
Embodiment 3
[0053] Embodiment 3: the synthesis of the PEI derivative containing hydroxyl
[0054] 0.5 g of PEI (can be linear or branched, molecular weight 2000) is dissolved in 5 ml of methylene chloride and placed in a 50 ml reaction bottle, a certain amount of cross-linking agents L2 and L5 (or one of L1-L4) One or two kinds, quantified according to the molar ratio of the reaction) dissolved in 5ml of dichloromethane at 20-25°C, added dropwise to the above-mentioned reaction flask under magnetic stirring, and then continued to react for 6 hours, and the reaction solution was evaporated by rotary evaporation to make it The volume is reduced by more than half, and then dialyzed for more than 72 hours, and the hydroxyl-containing PEI derivative product is obtained after freeze-drying. Various hydroxyl-containing PEI derivatives (raw materials are shown in Table 1; Table 2), various reaction combinations and the resulting The polymer structure is schematically shown in Figure 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com