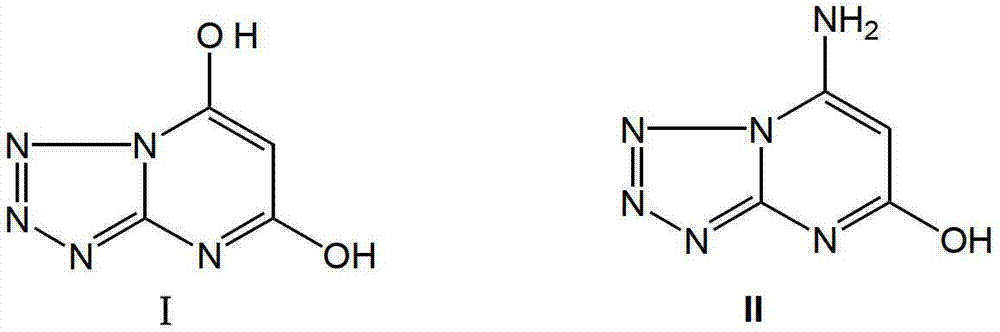Compound I tetrazole (1, 5-a) pyrimidine-5, 7-diol and synthetic route thereof
A synthetic route and compound technology, applied in organic chemistry, drug combination, antiviral agents, etc., can solve problems such as poor selectivity, toxicity, and high toxicity of systemic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
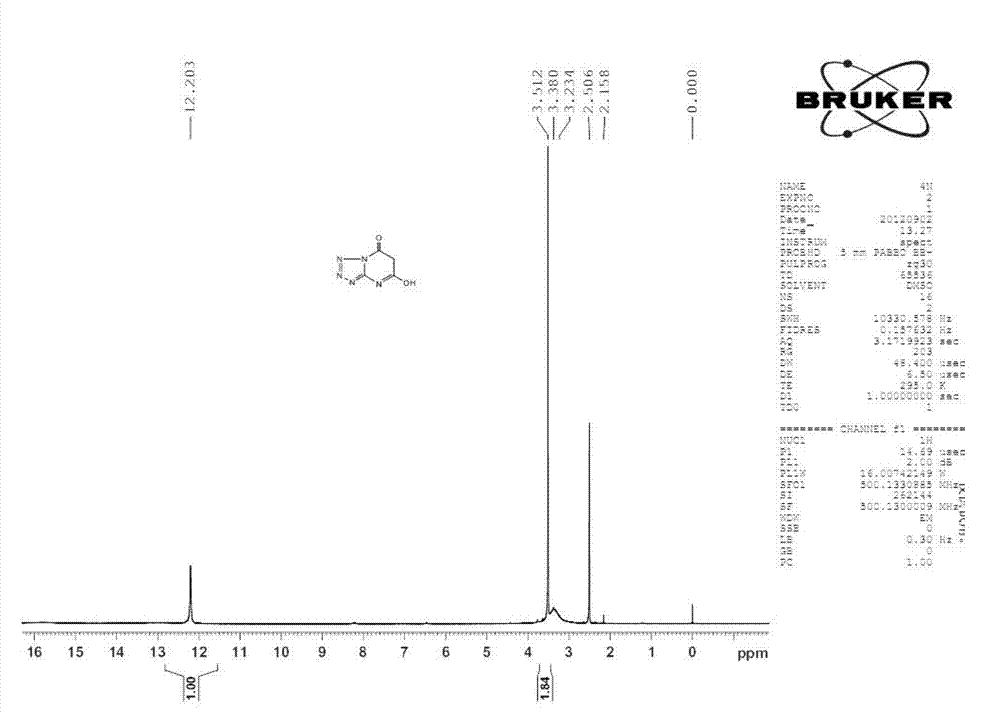
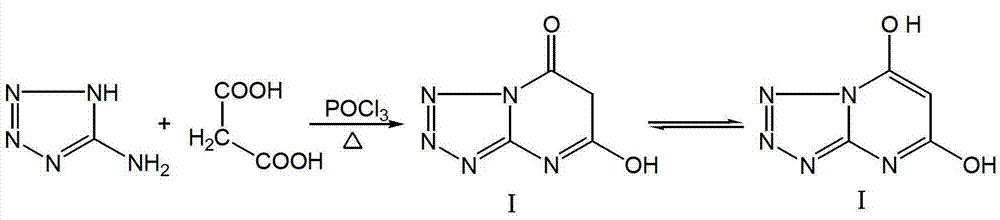
[0022] 1. Preparation of tetrazo[1,5-a]pyrimidine-5,7-diol (compound I) (Route 1)
[0023] Add 3g of 5-aminotetrazole, 3.7g of malonic acid and 45mL of chloroform into a round-bottomed flask, heat to reflux, and after the solid is uniformly dispersed, add 10.8g of phosphorus oxychloride, heat to reflux and stir overnight. Evaporate the solvent in a water bath, add ice water, stir well, and filter with suction to obtain a solid. The obtained solid was heated with water, refluxed, and then an appropriate amount of ethanol was added. After the solid was completely dissolved, it was filtered while it was hot. 4.3g, m.p.>300℃, yield 79.63%, elemental analysis C 4 h 3 N 5 o 2 . Found: C31.06, H2.12, N45.61; Calculated: C31.38, H1.98, N45.74.
[0024] 2. Test report on inhibition of cell division cycle phosphatase CDC25B by tetrazo[1,5-a]pyrimidine-5,7-diol (compound I)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com