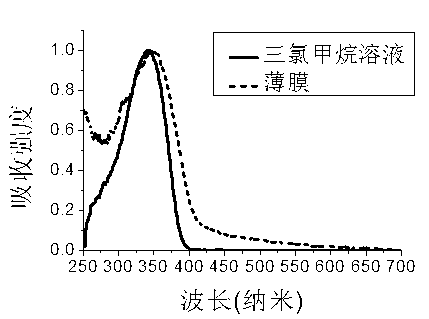
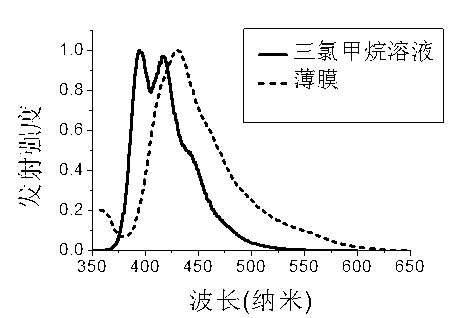
Fluorenyl organic framework material, preparation and application method thereof
An organic framework and fluorene-based technology, which is applied in the field of organic semiconductor materials, can solve the problems of no method for synthesis, no application reported, etc., and achieves the effects of cheap raw materials and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
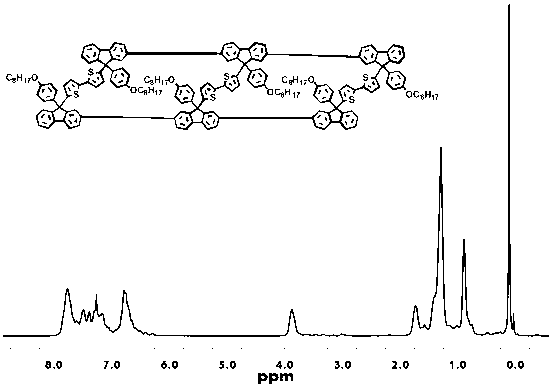
[0073] Example 1. Trimeric (bis(9-octyloxyphenyl-9-bithiophene)fluorene)
[0074]
[0075] 2-Bromo-9-p-octyloxyphenyl-fluoren-9-ol
[0076] 2-bromo-9-octyloxyphenyl-9H-fluoren-9-ol
[0077] Take p-octyloxybromobenzene (8.27g, 29mmol) and magnesium Mg (0.58g, 24mmol) to react to generate Grignard reagent, and dissolve 2-bromofluorenone (3.135g, 12.1mmol) in tetrahydrofuran (20mL) at 60 ℃ for 24 hours, a large amount of white precipitate was formed, and finally saturated NH 3 Aqueous Cl converts Grignard salts to alcohols. After the reaction was completed, it was extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether: dichloromethane (3:2) to obtain a slightly pale yellow oily tertiary alcohol (4.46 g, 90%).
[0078] GC-MS (EI-m / z): 464 / 466 (M + ).
[0079] 1 H NMR (400MHz, CDCl 3 ,ppm):δ7.65-7.65(d,J=7.2Hz,1H),7.64-7.45(m,3H),7.40-7.35(m,3H),7.32-7.24(m,5H),3.91-3.88 (t,J=7.6Hz,2H),2.47(s,1H),1.7...
Embodiment 2、 2
[0115] Example 2, bis(bis(9-octyloxyphenyl-9-(9-hexylcarbazole))fluorene)-(bis(9-octyloxyphenyl-9-bithiophene)fluorene)
[0116]
[0117] "H"-shaped oligomeric tetratertiary alcohol (0.2mmol, 0.385g) and 9-octylcarbazole (0.40mmol, 0.10g) were dissolved in 500ml of anhydrous dichloromethane (dried by anhydrous sodium sulfate in advance), and stirred rapidly In this case, a solution of boron trifluoride in ether dissolved in 50 ml of anhydrous dichloromethane (dried over anhydrous sodium sulfate in advance) was added dropwise. Stir at room temperature for 24h. After the reaction was completed, it was extracted with dichloromethane, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (4:1) to obtain a white solid powder (65.61 mg, 15%).
[0118] MALDI-TOF-MS(m / z):2969 / 2970[M + ].
[0119] 1 H NMR (400MHz, CDCl 3,ppm):δ7.98(br,ArH),7.91(br,ArH),7.81(br,ArH),7.78(br,ArH),7.75(br,ArH),7.73(br,ArH),7.66( b...
Embodiment 3、 2
[0128] Example 3, Bis(bis(9-phenyl-9-bithiophene)fluorene)-(bis(9-octyloxyphenyl-9-bithiophene)fluorene)
[0129]
[0130] 2-Bromo-9-phenyl-fluoren-9-ol
[0131] 2-bromo-9-phenyl-fluoren-9-ol
[0132] Take bromobenzene (4.48g, 29mmol) and magnesium Mg (0.58g, 24mmol) to react to generate Grignard reagent, and react with 2-bromofluorenone (3.135g, 12.1mmol) dissolved in tetrahydrofuran (20mL) at 60°C for 24 hours , generate a large amount of white precipitate, and finally add saturated color NH 3 Cl converts Grignard salts to alcohols. After the reaction was completed, it was extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly pale yellow solid tertiary alcohol (3.70 g, 90%).
[0133] GC-MS (EI-m / z): 334 / 336 (M + ).
[0134] 1 H NMR (400MHz, CDCl 3 ,ppm):δ7.657-7.655(d,J=7.2Hz,1H),7.638-7.449(m,3H),7.403-7.35(m,3H),7.32-7.235(m,5H),2.512(s ,1H). 13 C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com