Electroluminescent material and application thereof
An electroluminescent material, a secondary substitution technology, applied in the direction of luminescent materials, circuits, electrical components, etc., to achieve the effect of good thermal stability, good thermal stability, and high triplet energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
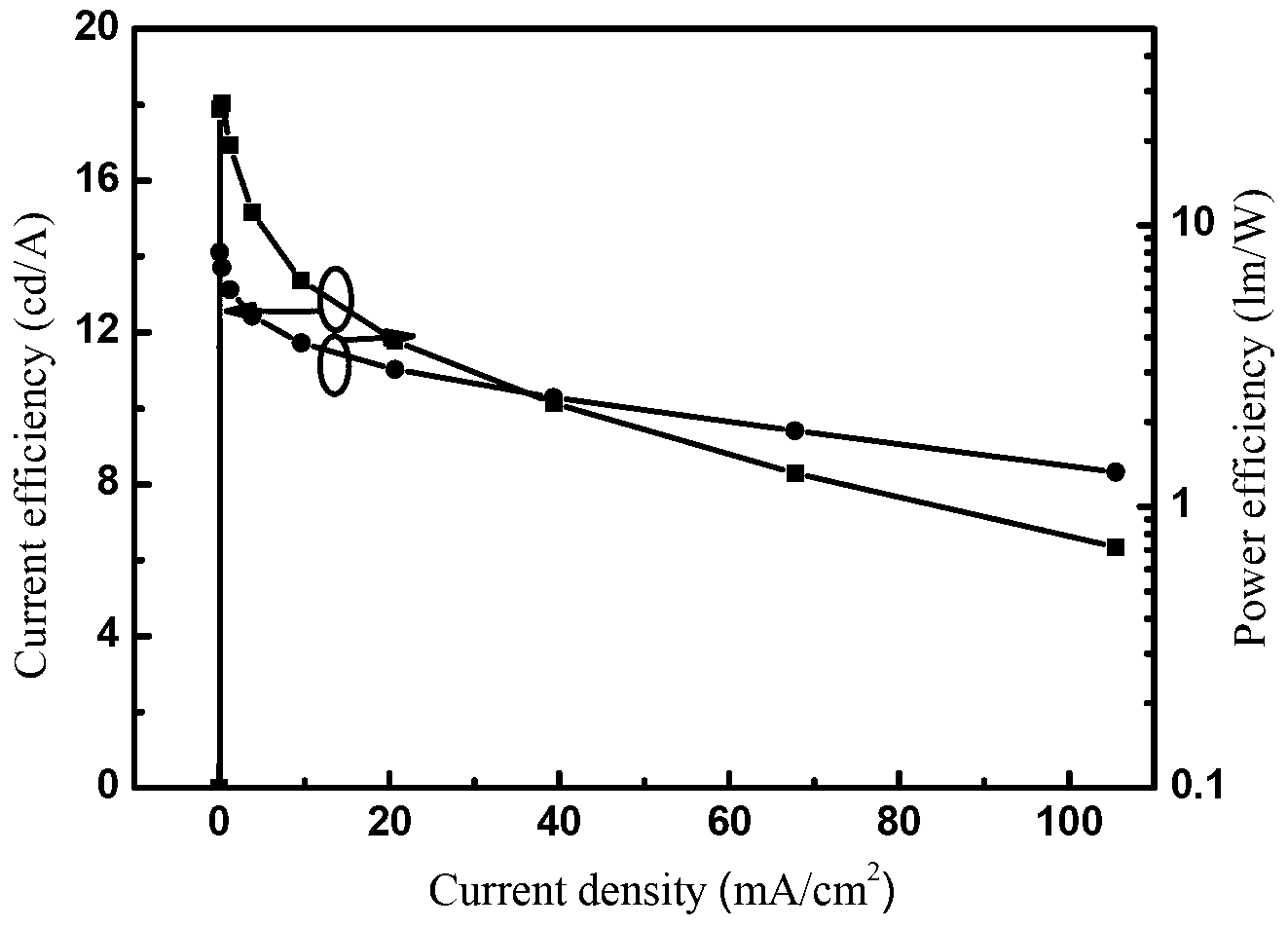
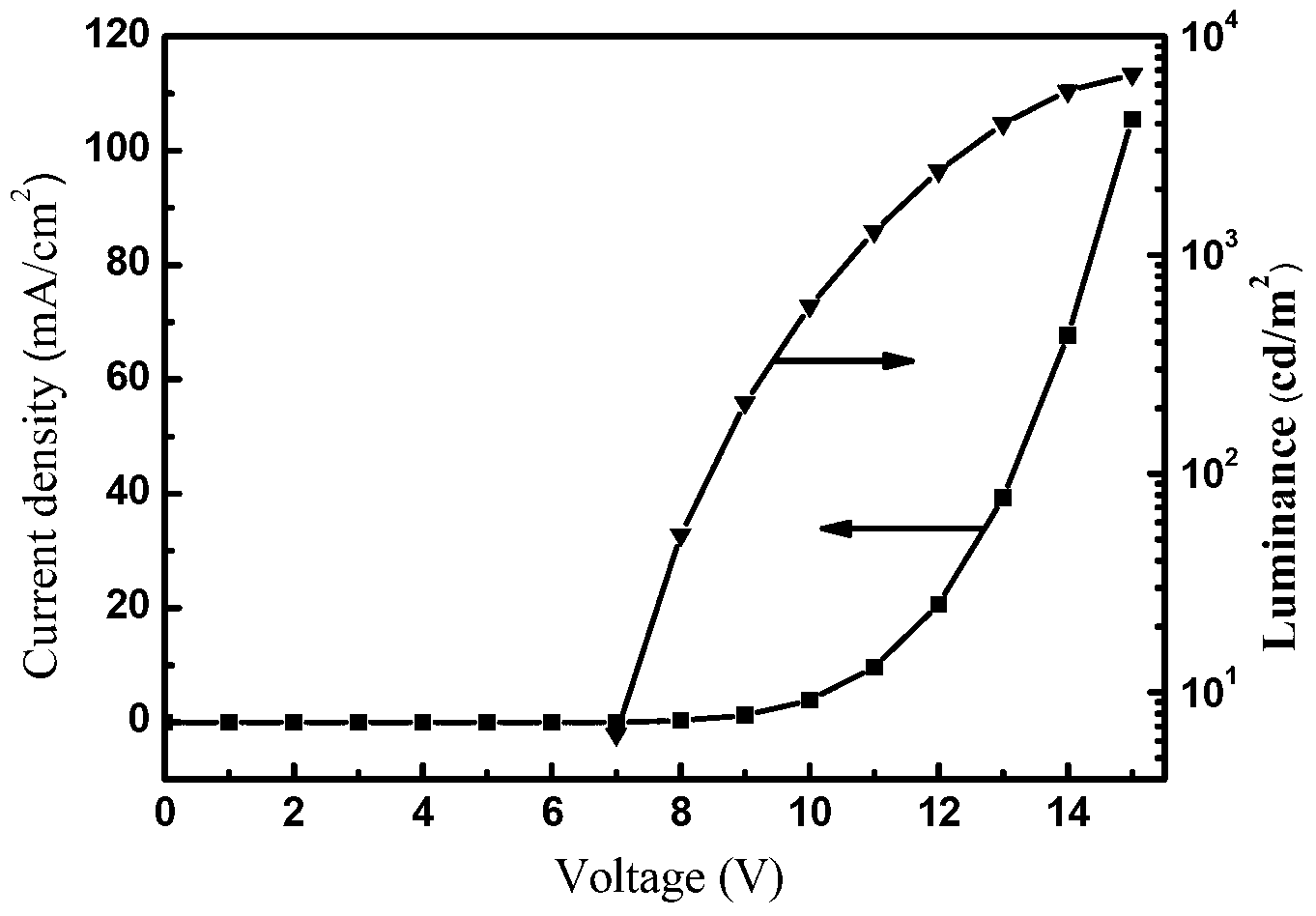
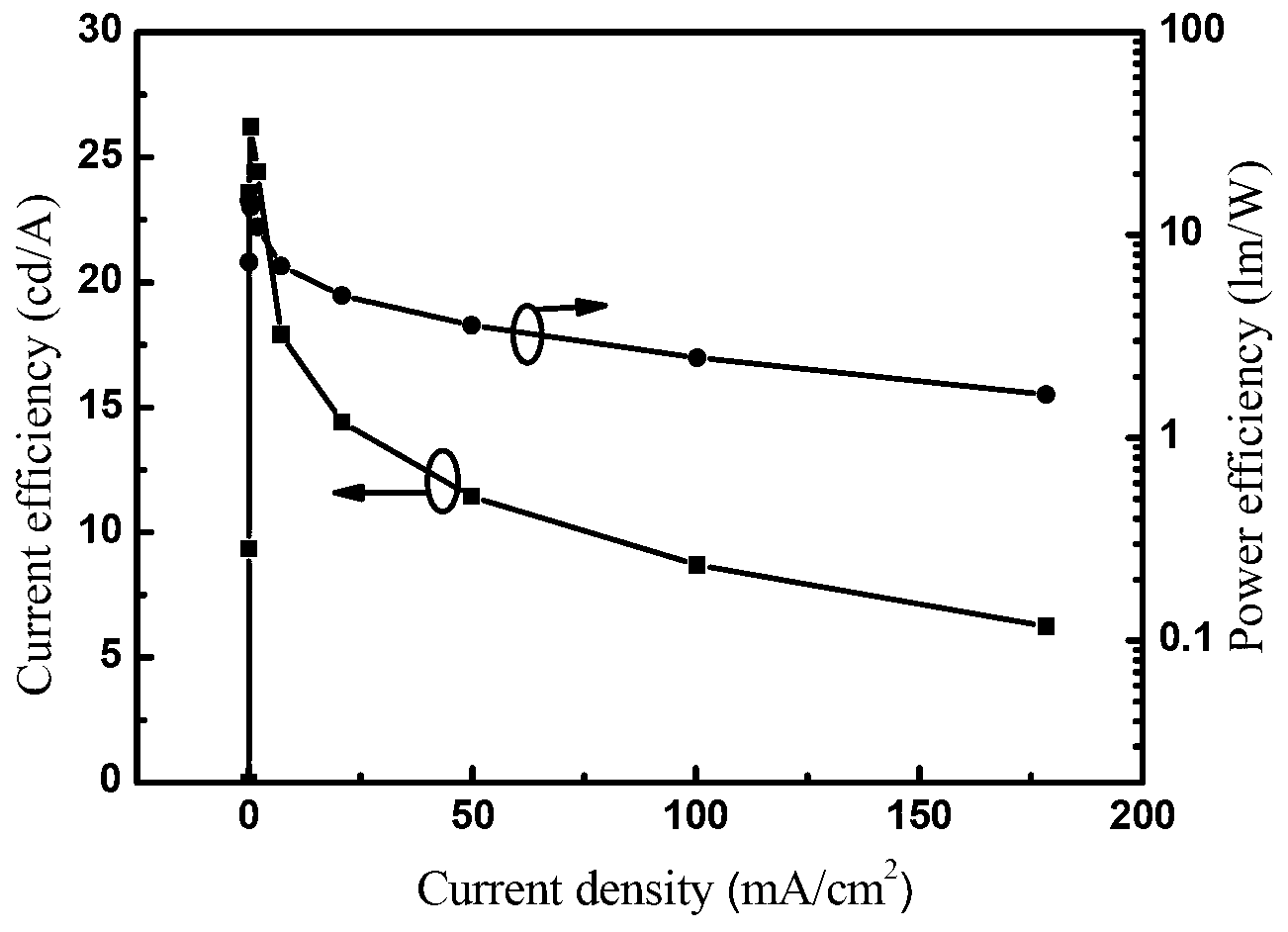
Image
Examples
Embodiment 1
[0059] Example 1: Synthesis of 1,3-bis[3-(9-diphenyltriazine)carbazole]benzene (compound 2, DTCZP)
[0060]
[0061] Step 1: Mix 3-bromocarbazole (12.3g, 0.05mol), 3,5-diphenyliodotriazine (21.54g, 0.06mol), potassium tert-butoxide (11.2g, 0.1mol), Toluene (150g) was added to the three-neck flask in turn, nitrogen gas was passed through the reaction flask for 30 minutes, then cuprous iodide (1.9g, 0.01mol) and o-phenanthroline (0.9g, 0.005mol) were added, and the reaction was refluxed for 8h to stop the reaction. After cooling down to room temperature, filter the filter cake with 200mL H 2 0. Rinse with 150mL o-xylene to obtain 17.88g of white solid, yield 75%. MS [m / z]: [M + ]=476.12,478.14.
[0062] Step 2: Mix 3-bromo-9-(3,5-diphenyltriazine)carbazole (9.54g, 0.02mol), m-diphenylboronic acid (1.66g, 0.01mol), potassium carbonate (4.14g, 0.03 mol), DMF (100mL) were added to the three-necked flask in turn, and Pd (PPh 3 ) 4 (0.23g, 0.2mmol), heated to reflux for 12h,...
Embodiment 2
[0063] Example 2: Synthesis of 1,4-bis{4-[5-(3,5-diphenyltriazine)benzofuran]}benzene (compound 19, DTBFuP)
[0064]
[0065] Step 1: Mix 4-benzofuranboronic acid (2.55g, 0.012mol), 3,5-diphenyliodotriazine (3.59g, 0.01mol), potassium tert-butoxide (2.24g, 0.02mol), o Pd(PPh 3 ) 4 (0.12g, 0.1mmol), reflux reaction for 6h, TLC showed that the reaction of raw materials was complete, and the reaction was stopped. Suction filtration, the filtrate is spin-dried and column chromatography obtains light yellow solid 3.39g, yield 85%, MS [m / z]: [M + ]=399.28.
[0066] Step 2: Add TBFu (2.39g, 6mmol) and THF (30g) into a three-necked flask, blow nitrogen gas to cool down to -78°C, add n-butyl lithium in n-hexane solution (3.27mL, 7.2mmol) dropwise, and add dropwise over 10 minutes Finished; keep at -78°C for 30 minutes, then raise the temperature to -30°C for 2 hours; then cool down to -78°C and add trimethyl borate (1.25g, 12mmol) dropwise, and add dropwise for 5 minutes; warm u...
Embodiment 3
[0068] Example 3: Synthesis of DTCZQu (Compound 56)
[0069]
[0070] Step 1: Add 4-bromo-2-iodonitrobenzene (32.8g, 0.1mol), 1-naphthaleneboronic acid (18.92g, 0.11mol), toluene (200g), methanol (200g) into the three-necked flask in sequence, and then Potassium carbonate (27.6g, 0.2mol) was added to the reaction flask as a 2M aqueous solution. Add Pd(PPh 3 ) 4 (1.2g, 1mmol), reflux reaction for 6h, TLC showed that the reaction of raw materials was complete, and the reaction was stopped. Add 100g of ethyl acetate to the reaction system, extract and separate, wash the organic phase with 100g of water and 100g of saturated brine respectively, dry with anhydrous sodium sulfate and spin dry, obtain 20.3g of light yellow solid by column chromatography, yield 62%, MS [m / z]: [M + ]=327.07.
[0071] Step 2: Add DTCZQu-A (19.68g, 0.06mol) and o-dichlorobenzene (200g) into a three-neck flask, blow nitrogen gas to raise the temperature to reflux, keep the reaction for 24h, cool d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com