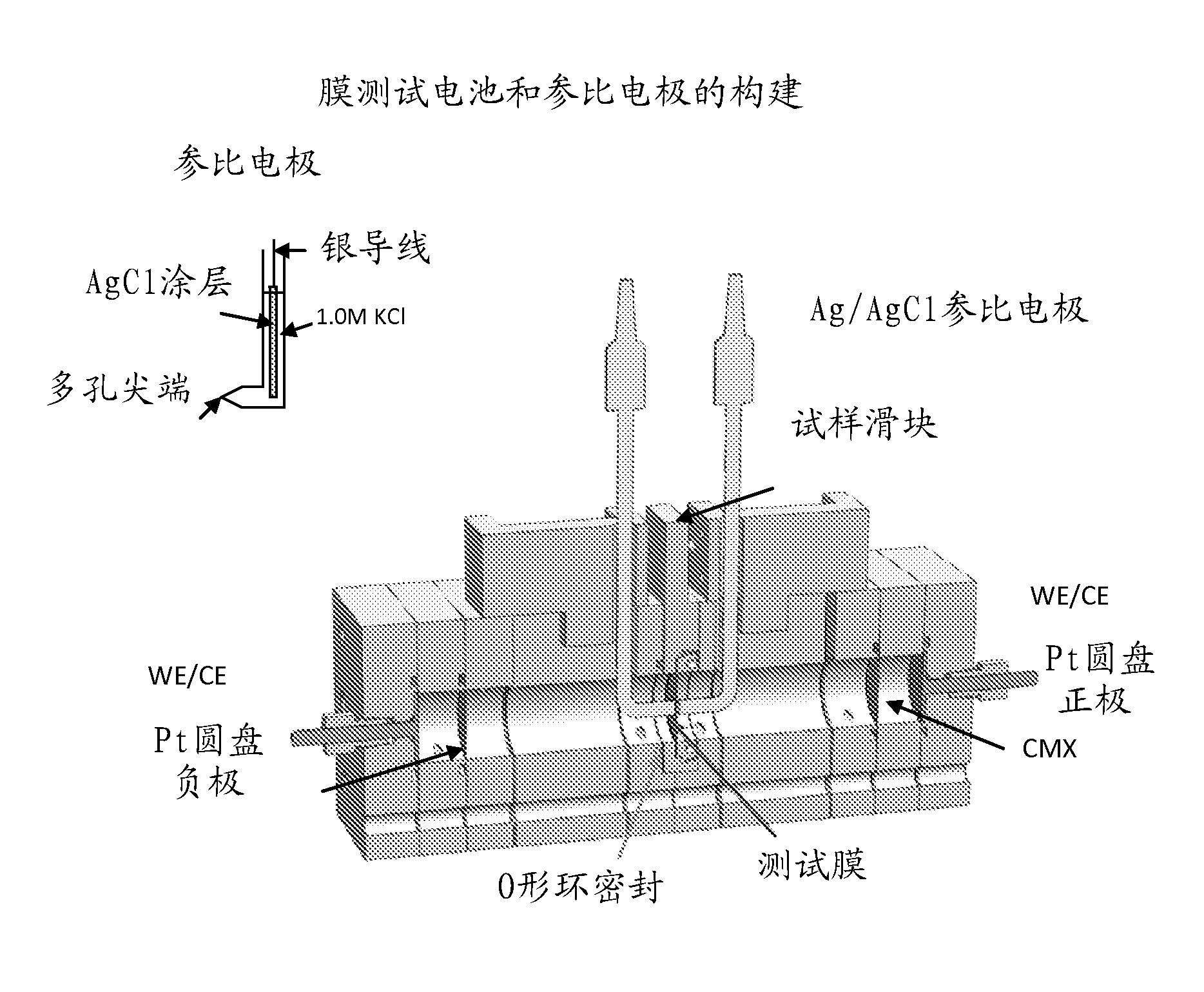
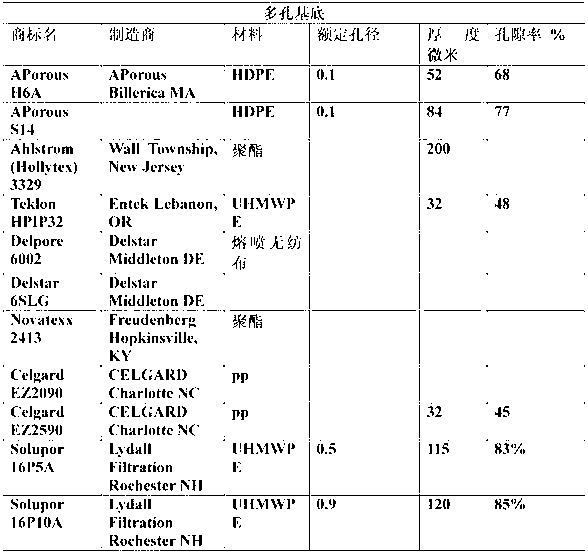
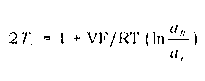Process for making monomer solution for making cation exchange membranes
A monomer solution and solution technology, applied in the direction of final product manufacturing, chemical instruments and methods, sustainable manufacturing/processing, etc., can solve problems such as difficult ultra-high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Preparation of styrene sulfonic acid pyridine and CEM
[0080] In an 8 oz jar, 61.1 g of NaSS, 29.92 g of pyridine hydrochloride, 44.38 g of NMP, 47.25 g of DVB-80, and 0.42 g of MEHQ were added. The mixture was heated to 76°C and refluxed for 100 minutes, then slowly cooled under stirring for 18 hours, and then filtered at 15°C using a vacuum pump suction. Output: 133.45 g of clear yellow-green solution. The filtered NaCl was dried in an oven at 90°C for 70 hours; weighing: 41.68 g.
[0081] 0.35 gm of Vazo-64 (DuPont) was added to 50.43 g of the solution obtained above. The samples of Teklon HPIP, APorous H6A, Celgard EZ2090, Celgard EZ2590, Solupor 10P05A and Solupor 16P10A were immersed for 45 minutes respectively, and then they were sandwiched between Mylar discs, and any bubbles in the sandwich were removed by squeezing. Place the interlayer in an aluminum weighing pan, and put the weighing pan into a Ziploc® or similar expandable plastic bag, pressurize with nitrog...
Embodiment 2
[0088] CEM prepared from a mixture of PySS, 2-SEM DVB, AA and NMP:
[0089] In a 40 ml glass bottle containing 7.38 g of acrylic acid, dissolve 7.6 g of NMP, add 7.4 g of DVB-80 and 0.15 g of Vazo-64 (DuPont) to obtain a completely transparent light yellow solution.
[0090] To 20.09 g of the solution from Example 1 doped with Vazo-64, 2.6 g of the above solution and 3.10 g of 2-SEM (2-sulfoethyl methacrylate, Evan Chemetics) were added. Stir at room temperature until homogeneous mixture. Then sandwich the samples of Tekion HPIP, APorous H6A, Celgard EZ2090, Celgard EZ2590, Solupor 10P05A and Solupor 16P10A between Mylar discs, and squeeze to remove any bubbles in the sandwich. The sandwich is placed in an aluminum weighing pan, and the weighing pan is placed in a Ziploc™ bag and pressurized with nitrogen. The Ziploc™ bag was placed in an oven at 90°C for 45 minutes.
[0091] Each film sample thus prepared was placed in 0.5N NaCl(aq) to adjust. The Solartron electrochemical teste...
Embodiment 3
[0096] Synthesis of styrene sulfonic acid pyridine (PySS)
[0097] Add 50.22 g of NaSS, 26.40 g of pyridine hydrochloride, and 26.30 g of NMP to an 8 oz jar. The solution was heated at 40-75°C for 6 hours under stirring and reflux, and then slowly cooled under stirring for 18 hours. The precipitated NaCl is filtered out using a vacuum pump suction device at room temperature. Output: 61 g of clear yellow-green solution. The filter cake weighed 31.10 g after being dried in an oven at 80°C for 6 days.
[0098] The clear yellow-green solution was kept in the refrigerator overnight, forming some flaky crystals. The crystals were filtered using a vacuum pump suction device; 58.5 g of remaining solution and 2.6 g of precipitated crystals were obtained.
[0099] In this example, 0.244 mol of NaSS was initially present. If MW = 183.2 in terms of sulfonate ion, then there is 73% sulfonate in 61.1 g of the final clear solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com