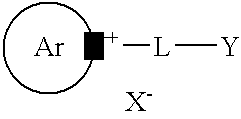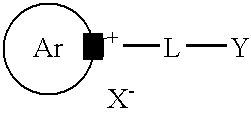Mono and bis-ester derivatives of pyridinium and quinolinium compounds as environmentally friendly corrosion inhibitors
a technology of pyridinium and quinolinium compounds, which is applied in the field of new compounds, can solve the problems of inability to readily biodegrade compounds and often unacceptable biotoxicity profiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045]Epichlorohydrin (34 g, 0.36 m) is heated at 80° C. with a catalytic amount of concentrated hydrochloric acid (1 ml) for 20 minutes to open the ring and obtain 1-chloro-2,3-dihydroxy-propane. This is condensed with 1.5 equiv. of tall oil fatty acids (155 g, 0.55 m) at 150° C. for 3 hours to obtain a mixture of mono and bis esters. Any residual hydrochloric acid is neutralised with solid sodium bicarbonate. Substituted pyridine (Alkolidine 12, available from Lonza Ltd., Basel, Switzerland, 95 g, 0.55 m) is added and the reaction mixture is heated at 150° C. for 2 hours to obtain the corresponding substituted pyridinium salt. Sodium chloride is filtered off and any other inorganics are removed by dissolving in water. Yield: 260 g.
example 2
[0046]Chloroacetic acid (20 g, 0.211 m) is heated with n-octanol (28 g, 0.22 m) at 120° C. for 2 hours in the presence of a catalytic amount of concentrated hydrochloric acid. The reaction mixture is allowed to cool and substituted pyridine (Alkolidine 12, 42 g, 0.22 m) is added. The reaction mixture is heated at 130° C. for another 2 hours to provide the substituted alkyl pyridinium ester. Yield 86 g.
example 3
Performance Testing
[0047]Standard Linear Polarisation Resistance (LPR) techniques in a ‘bubble’ or stirred kettle assembly are used to measure the instatanteous corrosion rate in the brine solution as a function of time. Synthetic seawater (deionised water containing 3% sodium chloride) saturated with CO2 and de-aromatised kerosene (LVT-200) are used in a 90:10 ratio at 60° C.
[0048]An automated electrochemical measurement system (ACM Instruments Gill 12) and associated software is used to conduct electrochemical measurements. Three pin probes constructed from type C1018 mild steel are used for all bubble test and Rotating Cylinder Electrode (RCE) measurements. Standard ranges of concentrations are examined to generate performance concentration curves in an environment where the inhibitor species has been allowed to partition. A blank corrosion rate for each cell is measured every 10 minutes for two hours whereupon chemical injection is made into the hydrocarbon phase. The corrosion ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com