Yinhuang medicine for clearing away lung-heat and preparation method thereof
A silver-yellow lung-clearing and drug technology, applied in the directions of drug combination, pharmaceutical formula, plant raw material, etc., can solve the problems of high production cost, long period, prolonged healing period of chronic bronchitis, etc., achieve good curative effect and increase the content of Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
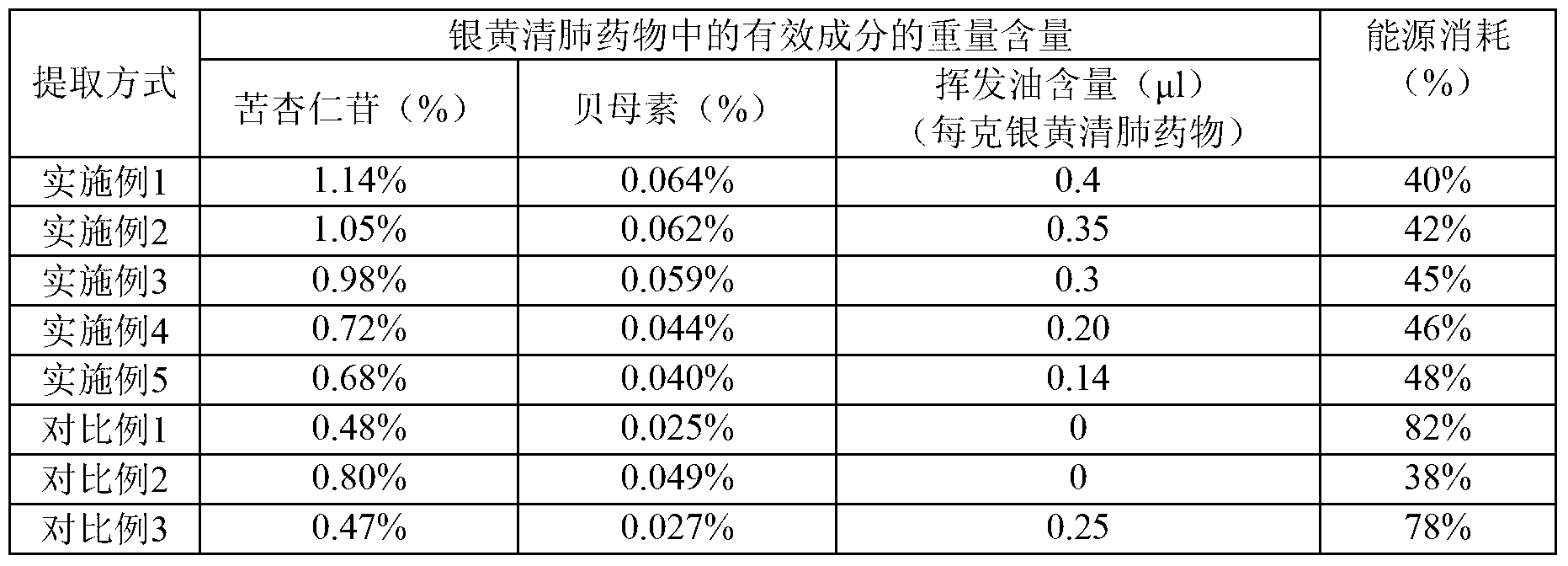
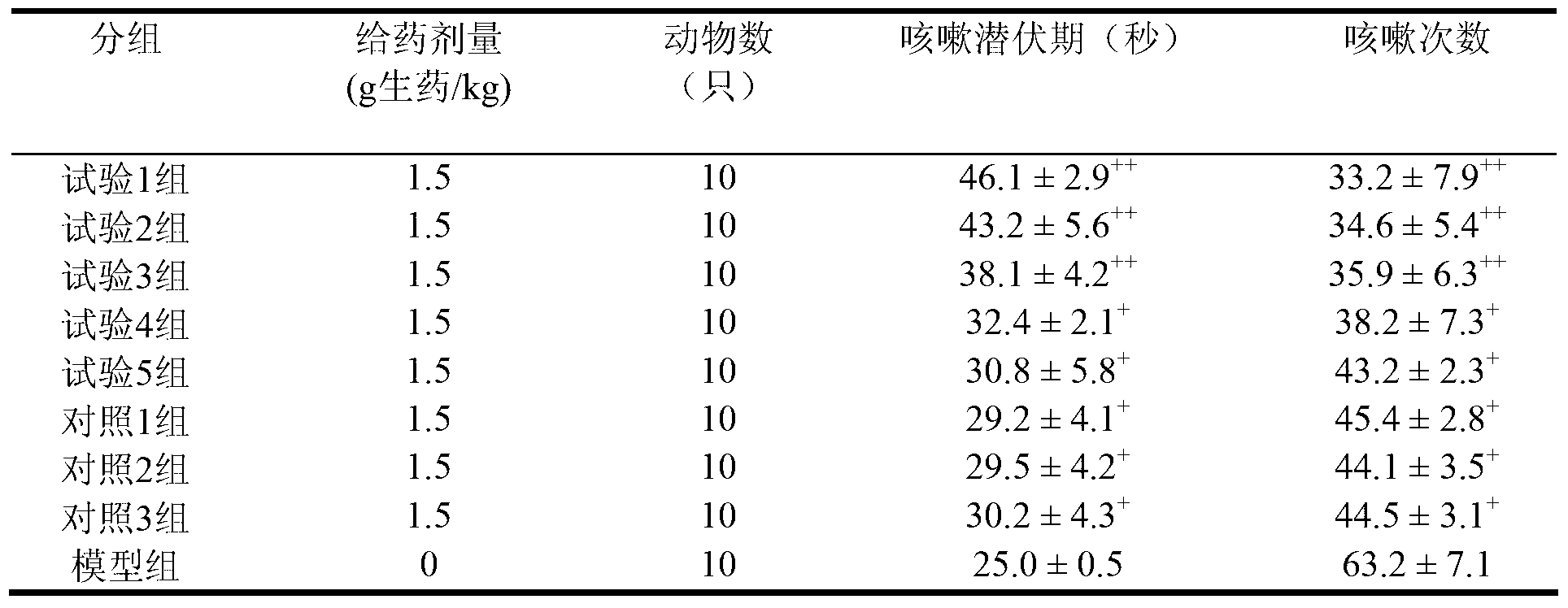
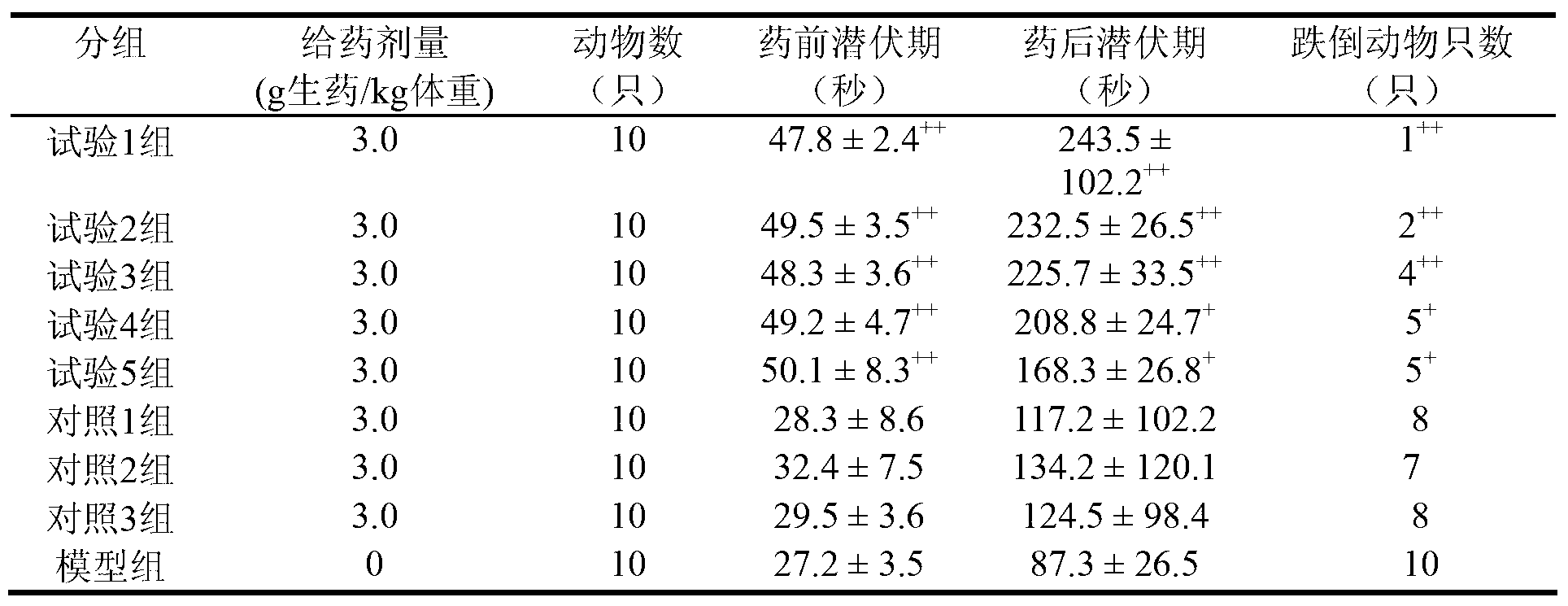
[0017] Due to the poor curative effect of the chronic bronchitis medicine prepared by the existing technology and the long preparation period, in order to solve the above problems, the present invention provides a preparation method of Yinhuang Qingfei medicine, which includes the following steps: S1, taking ephedra or roasting Ephedra is pulverized to obtain ephedra powder or roasted ephedra powder for later use; S2, using steam distillation to extract volatile oil from Citrus aurantium, loquat leaves and Shichangpu to obtain volatile oil, extract and extraction residue; use clathrate technology to extract volatile oil Inclusion to obtain clathrate; S3, using dynamic continuous extraction process to extract Tinglizi, bitter almond, fritillary fritillaria and Schisandra chinensis, to obtain alcohol extract and residue; concentrate the alcohol extract to obtain alcohol extract ; S4. Mix the extraction residue, alcohol extraction residue, Folium Isatidis Folium, Pangosia chinensi...
Embodiment approach
[0027] In addition, in addition to the advantages of the preparation process, the composition itself of the present application also has advantages. According to a typical embodiment of the present invention, in parts by weight, the raw material of the Yinhuang lung-clearing drug includes the following components: 55-65 parts of Tinglizi; 35-40 parts of ephedra or roasted ephedra; 40 parts of bitter almond ~50 parts of Fritillaria 40~50 parts; loquat leaves 40~50 parts; Daqingye 27~32 parts; Shichangpu 40~50 parts; 50 parts; 12-18 parts of schisandra; 12-18 parts of Citrus aurantium; 55-65 parts of raw gypsum; 12-18 parts of licorice.
[0028] Using the above raw material formula, the prepared Yinhuang Qingfei drug has a good therapeutic effect. Among the traditional Chinese medicine ingredients, Fritillaria, loquat leaves, Shichangpu relieve cough and reduce phlegm, ephedra relieves lung and relieves asthma, raw gypsum clears lung heat, Daqingye clears away heat and detoxifi...
Embodiment 1
[0035] 1) The raw materials are as follows: Tinglizi 60g; Ephedra 37.5g; Bitter almond 45g; Fritillaria 45g; Loquat leaves 45g; ; Citrus aurantium 15g; Gypsum 60g; Licorice 15g.
[0036] 2) Weigh the above-mentioned fourteen traditional Chinese medicines according to the formula, and grind the ephedra into ephedra powder with a particle size of 125 μm, and set aside. Put Citrus citrus Fructus Fructus, loquat leaves and Shichangpu into the first extraction tank, add 5 times the amount of water, and co-distill for 6 hours to obtain the volatile oil extract and extraction residue; use β-cyclodextrin at 40 ° C to extract The clathrate was carried out for 2 hours, and dried at 60° C. for 2 hours to obtain the clathrate.
[0037] 3) Mix Tinglizi, bitter almond, Fritillaria and Schisandra to obtain a mixture; add 7 times the amount of ethanol with a mass concentration of 70% to the mixture, turn on the steam to heat and reflux, turn on the dynamic pump to start pressurization, and u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com