Ophthalmic solution containing diquafosol, method for producing same, and method for preventing formation of insoluble precipitate
A technology for diquafosol and eye drops, applied in the field of eye drops and preparation thereof, can solve the problems of increased preparation cost, clogging of filter filters, inability to remove precipitates, etc., and achieves stable physical and chemical properties and enhanced preservation efficiency. , excellent preservation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
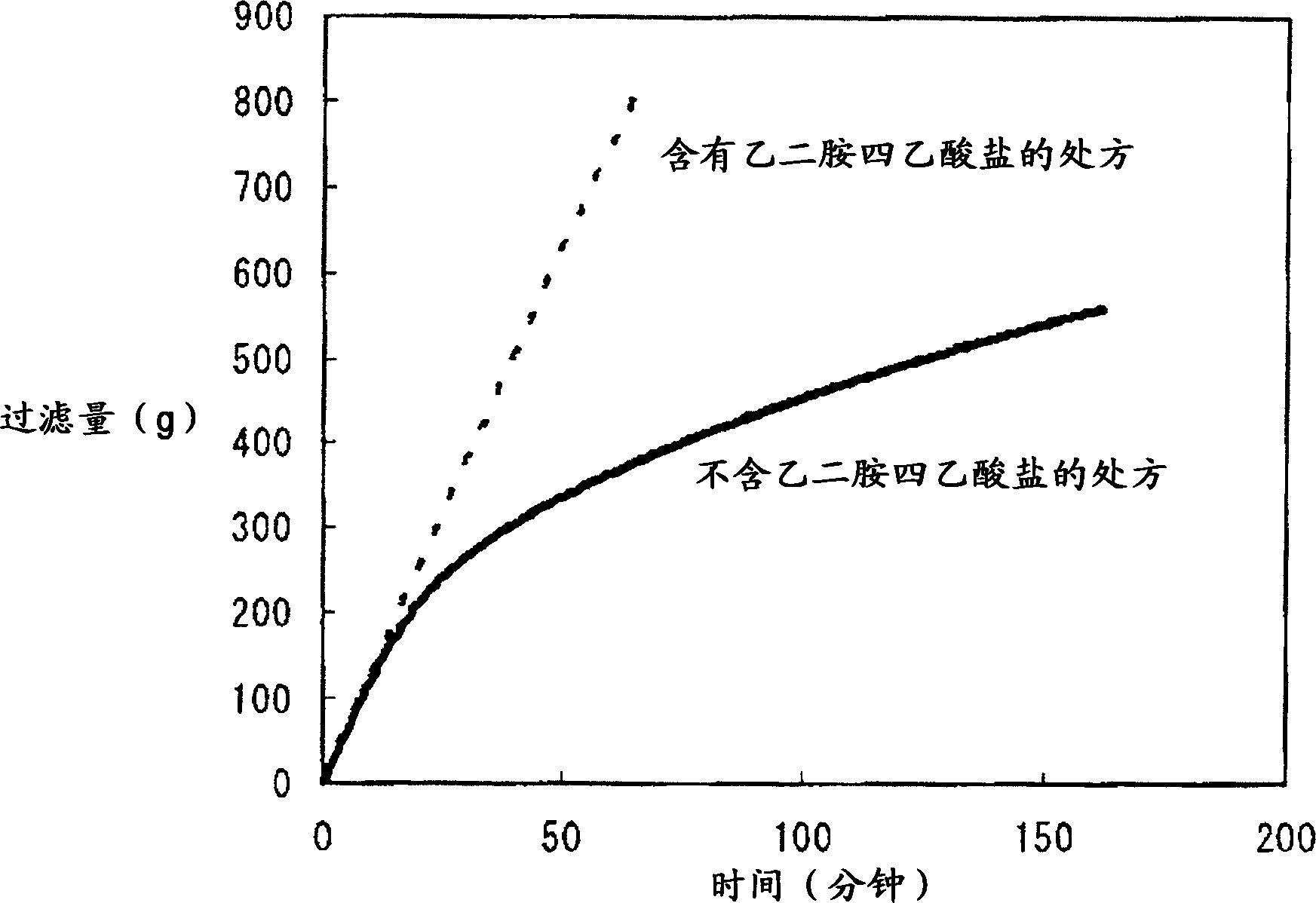
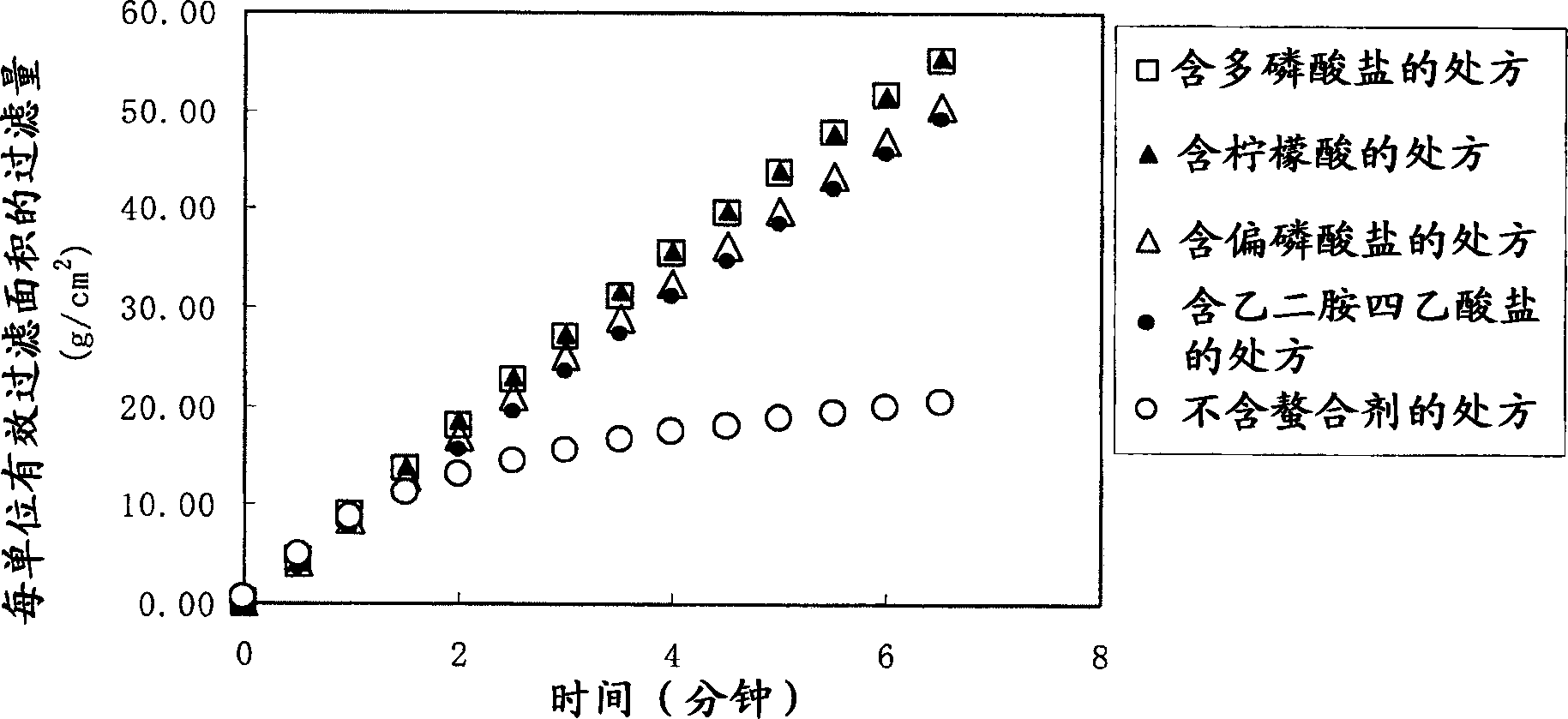
[0054] [Storage Stability Test]
[0055] Visually confirm whether there is any change in the properties of Diquafosol Eye Drops during storage, and at the same time study the effect of EDTA as a chelating agent on the change in properties.
[0056] (sample preparation)
[0057] · Prescriptions without EDTA
[0058] Dissolve 3 g of diquafosol sodium, 0.2 g of disodium hydrogen phosphate hydrate, 0.41 g of sodium chloride, 0.15 g of potassium chloride, and 0.0075 g of benzalkonium chloride in water to make it 100 mL, add a pH regulator to adjust the pH is 7.5, and the osmotic pressure ratio is 1.0.
[0059] Prescriptions containing 0.001 or 0.1% (w / v) EDTA
[0060] Dissolve 3 g of diquafosol sodium, 0.2 g of disodium hydrogen phosphate hydrate, 0.41 g of sodium chloride, 0.15 g of potassium chloride, 0.001 g or 0.1 g of sodium edetate hydrate and 0.002 g of benzalkonium chloride Make it 100 mL in water, and add a pH adjuster so that the pH becomes 7.5 and the osmotic pressur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com