Electro-catalysis oxygen reduction catalyst based on boron-nitrogen co-doped nano-diamond
A nano-diamond and co-doping technology is applied in the field of electrochemistry to achieve the effects of rich content, easy large-scale application, and improved electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1, boron nitrogen co-doped nano-diamond electrocatalytic oxygen reduction activity
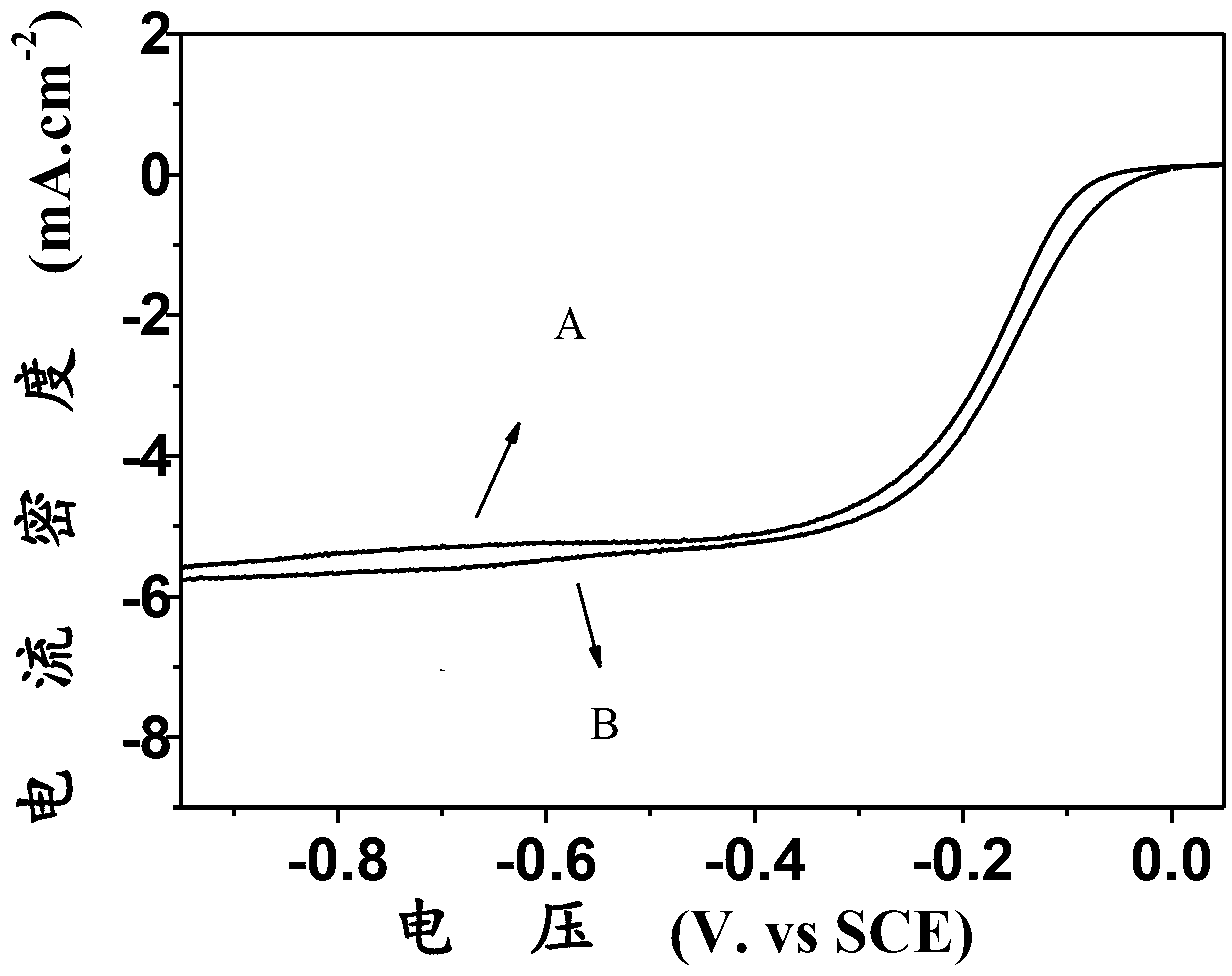
[0022] Using boron-nitrogen co-doped nano-diamond as the working electrode, Pt as the counter electrode, and a saturated calomel electrode as the reference electrode, in O 2 The linear voltammetry curve was measured at 1800 rpm in saturated 0.1M KOH. The preparation parameters of boron-nitrogen co-doped nano-diamonds are: CH 4 The volume fraction is 1.5%, N 2 Volume fraction 1.3%, B 2 h 6 The concentration is 10000ppm, the pressure is 5.5KPa, and the deposition is at 550°C for 6h. attached by figure 2 It can be seen that the onset voltage of oxygen reduction of boron-nitrogen co-doped nanodiamond is -0.04V, which is close to that of the commercial Pt / C catalyst (0.01V). At 1800rpm, its current density is 5.12mA.cm at -0.40V -2 , which can be compared to the commercial Pt / C catalyst (5.25mA.cm -2 ). This indicates that boron-nitrogen co-doped nanodiamond is a highly act...
Embodiment 2
[0023] Example 2, boron and nitrogen co-doped nano-diamond electrocatalytic oxygen reduction efficiency
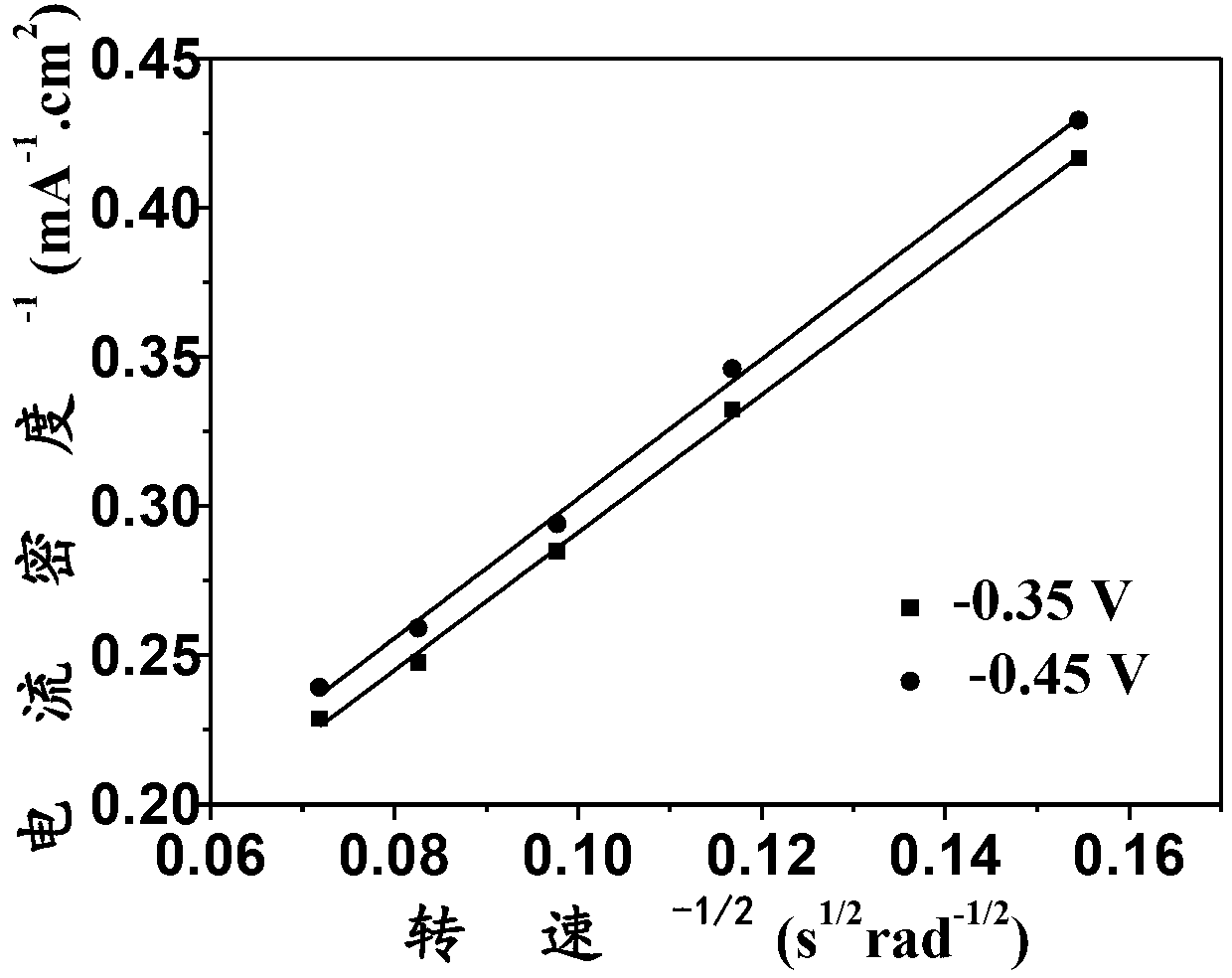
[0024] Using boron-nitrogen co-doped nano-diamond as the working electrode, Pt as the counter electrode, and a saturated calomel electrode as the reference electrode, in O 2 The linear voltammetry curves at different rotational speeds were measured in saturated 0.1M KOH, and the electron transfer number of the oxygen reduction reaction was calculated according to the corresponding Koutechy-Levich curves. The preparation parameters of boron-nitrogen co-doped nano-diamonds are: CH 4 The volume fraction is 0.8%, N 2 Volume fraction 0.5%, B 2 h 6 The concentration is 15000ppm, the pressure is 4.3KPa, and the deposition is at 450°C for 18h. attached by image 3 It can be seen that the electron transfer number of oxygen reduction of boron-nitrogen co-doped nano-diamonds is 3.95, indicating that boron-nitrogen co-doped nano-diamonds can efficiently proceed in accordance with...
Embodiment 3
[0025] Example 3, boron nitrogen co-doped nano-diamond electrocatalytic oxygen reduction stability
[0026] Using boron-nitrogen co-doped nano-diamond as the working electrode, Pt as the counter electrode, and a saturated calomel electrode as the reference electrode, at -0.3V, O 2 Current-time curve measured in saturated 0.1M KOH. The preparation parameters of boron-nitrogen co-doped nano-diamonds are: CH 4 The volume fraction is 2.5%, N 2 Volume fraction 0.8%, B 2 h 6 The concentration is 20000ppm, the pressure is 7.5KPa, and the deposition is at 500°C for 12h. attached by Figure 4 It can be seen that the oxygen reduction current of boron-nitrogen co-doped nano-diamond is relatively stable, and the current only drops by 6.0% after a long-term operation of 20000s, which is significantly higher than the stability of commercial Pt / C catalysts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com