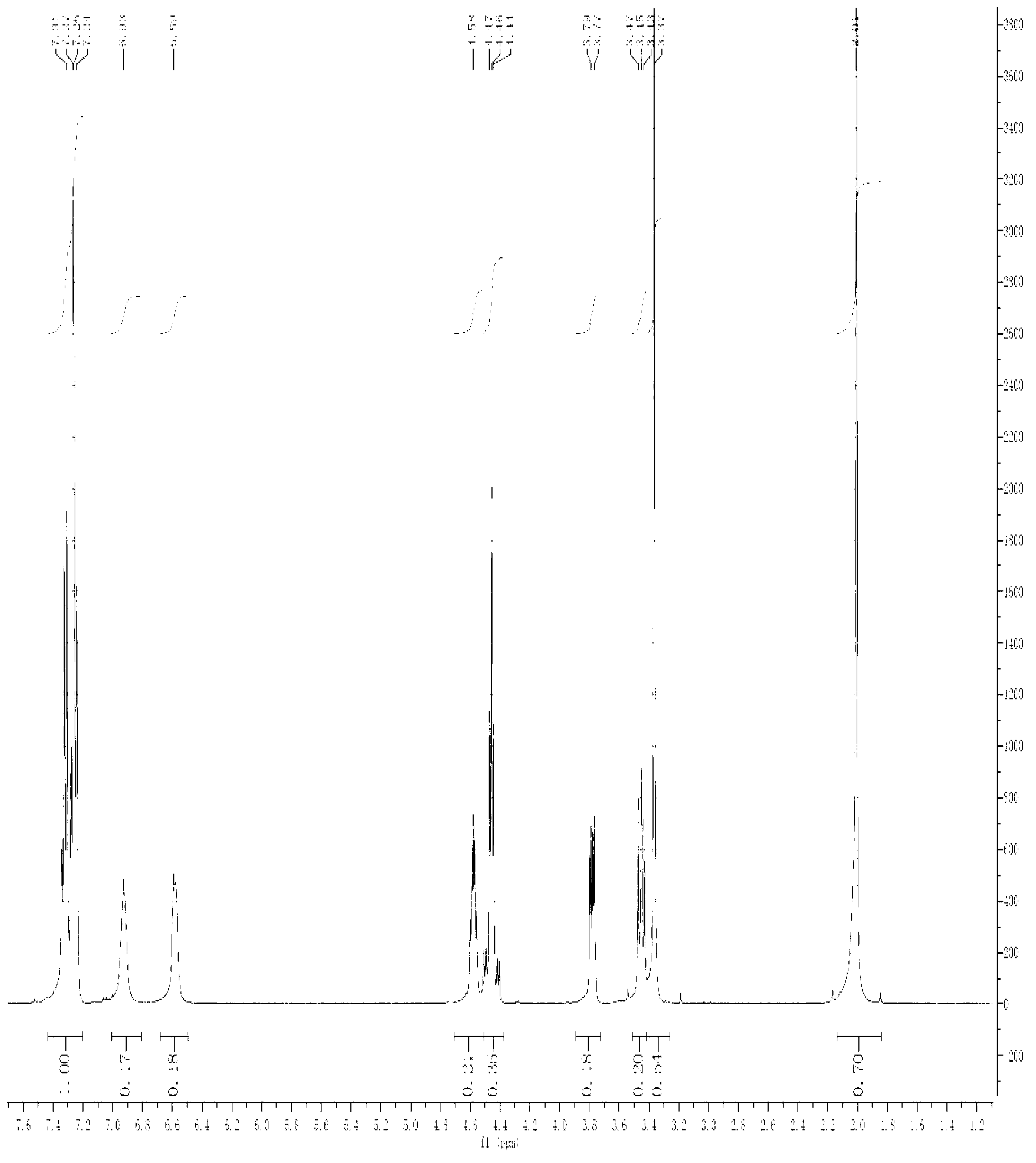
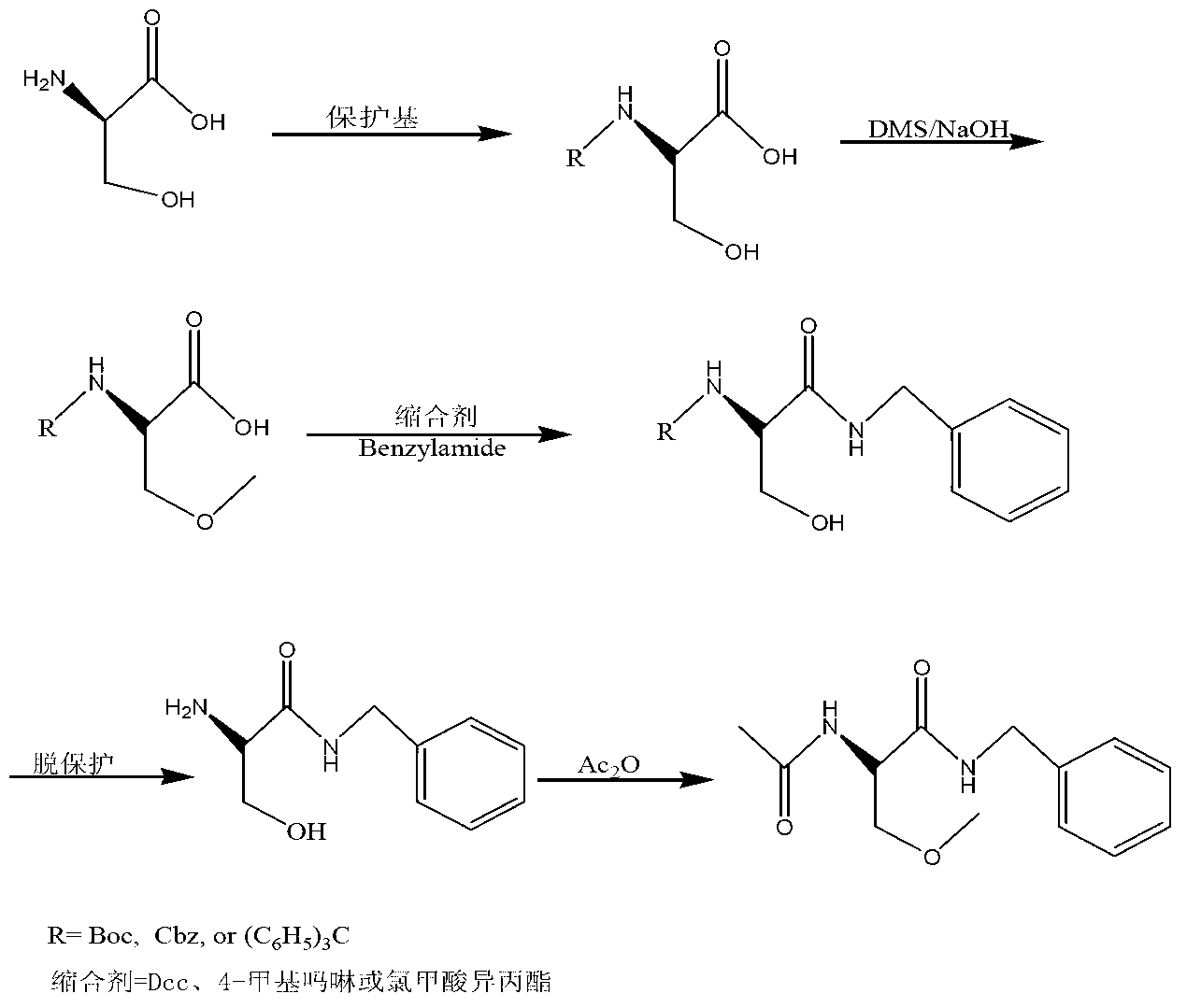
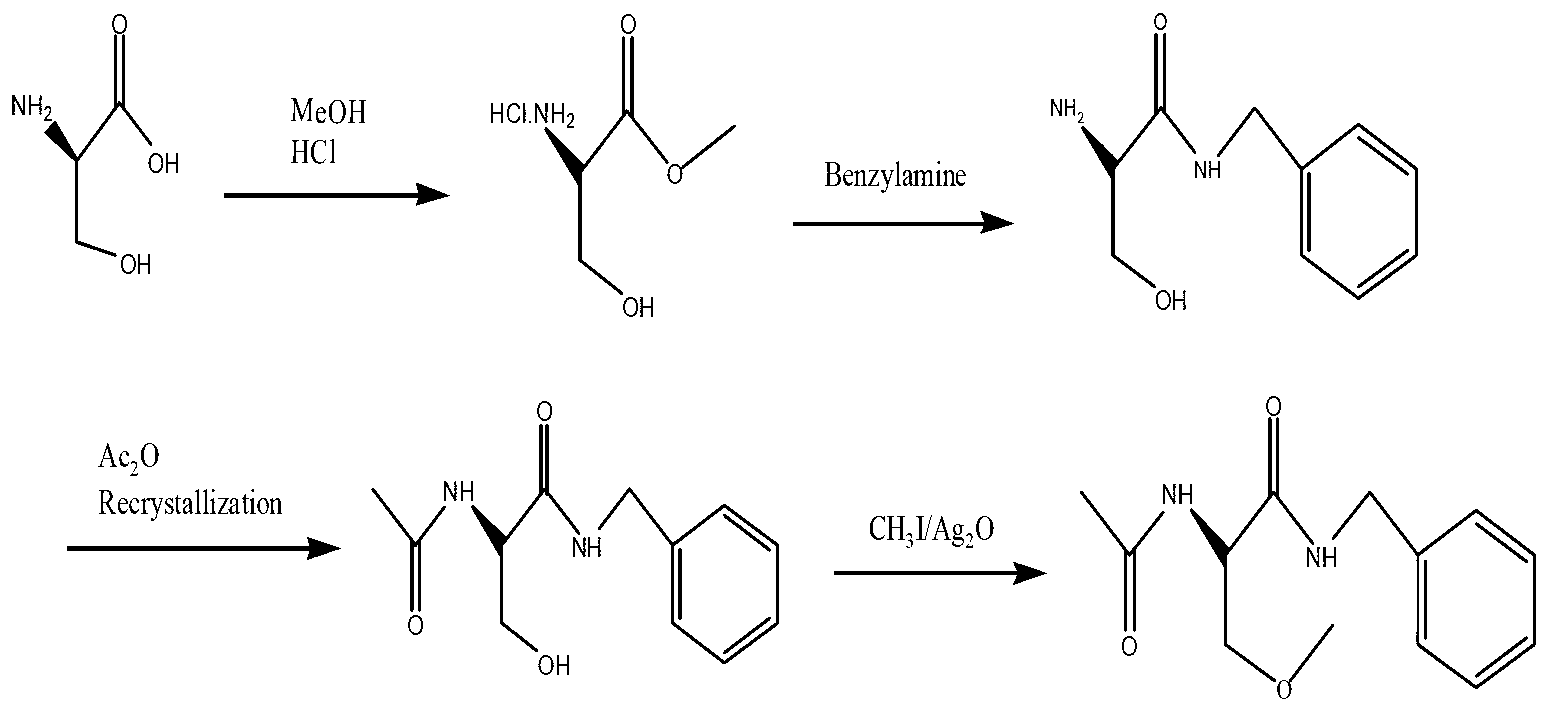
Lacosamide synthesis technology
A synthesis process, lacosamide technology, applied in the field of lacosamide synthesis process, can solve the problems of harsh conditions, complicated operation, high cost, etc., achieve the effect of optimizing reaction, optimizing process conditions and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) At 0°C, add 44.8g (0.57mol) of acetyl chloride to 250ml of anhydrous methanol, then add 20g (0.19mol) of D-serine (2), and heat up to 70°C for 7 hours under reflux after the addition. After the reaction was completed, it was concentrated to dryness at 45°C to a solid, then added acetone and stirred overnight, filtered the next morning, and dried to obtain 26.6 g of D-3-hydroxy-2-alanine methyl ester (3) as a white solid, with a yield of 90%. Melting point 163-164°C;
[0024] 2) At room temperature, dissolve 10g (0.064mol) of methyl D-3-hydroxy-2-alanine (3) in 40ml of acetone solution, then add 14.3g (0.141mol) of triethylamine and (BOC) 2 O14g (0.064mol) was added dropwise to the system, and reacted at room temperature for 10h. After the reaction was completed, acetone was evaporated under reduced pressure, water was added, extracted with ethyl acetate 3*50ml, the ethyl acetate layers were combined, and saturated hydrogen carbonate Wash with sodium 2*50ml, saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com