Lower alcohol detecting probe, preparation method and application thereof
A technology for detecting probes and lower alcohols, applied in the field of analytical chemistry detection, can solve the problems of expensive instruments, high technical requirements for operation and analysis, long detection time, etc., and achieves the effects of convenient processing, huge application prospects, and highly selective detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: R1 and R3 are hydrogen atoms, R2 are nitrogen atoms in the general formula, (probe 1 )
[0025] Synthesis steps: Add o-hydroxyaniline (0.011 mol) ethanol solution dropwise to 1,8-naphthyridine-2-carbaldehyde (0.01 mol) ethanol solution under nitrogen protection, stir at room temperature for 30 minutes and then reflux for 2 hours to remove Solvent, ethanol recrystallization, the final product (2.07 g, 65%) can be obtained.
[0026] The characterization is as follows: Characterization of 1 : Mp = 199?201 °C. HRMS (EI) m / z: calcd for C 15 h 11 N 3 ONa [M + Na] + , 272.0794; found, 272.0799. 1 H NMR (400 MHz, DMSO- d 6 , TMS): δ H 9.42 (s, 1H), 9.17 (d, 1H), 8.98 (s, 1H), 8.68 (m, 1H), 8.60 (m, 1H), 8.53 (m, 1H), 7.71 (m, 1H), 7.46 (m, 1H), 7.20 (m, 1H). 6.99 (m, 1H), and 6.92 (m, 1H). 13 C NMR (100 MHz, DMSO-d 6 ): δ C 159.24, 158.12, 155.85, 154.67, 152.47, 138.77, 137.98, 136.62, 129.57, 123.94, 123.61, 120.37, 120.14, and 117.04.
Embodiment 2
[0027] Embodiment two: R1 and R3 are hydrogen atoms in the general formula, and R2 is group, (probe 2)
[0028] The synthesis steps were the same as in Example 1, except that 8-ethyl acetate methoxyquinoline-2-carbaldehyde was used instead of 1,8-naphthyridine-2-carbaldehyde in Example 1, and the yield was 67%.
[0029] The characterization is as follows: Characterization of 2 : Mp = 159?160 °C. HRMS (EI) m / z: calcd for C 20 h 18 N 2 o 4 [M + Na] + , 373.1159; found, 373.1169. 1 H NMR (400 MHz, CDCl 3 , TMS): δ H 9.14(s, 1H), 8.38 (m, 1H), 8.25 (m, 1H), 7.50 (m, 1H), 7.42 (s, 1H), 7.26 (m, 2H), 7.04 (m, 2H), 6.96 (m, 2H), 5.02 (s, 2H), 4.31 (q, 2H). and 1.30 (t, 3H). 13 C NMR (100 MHz, CDCl 3 ): δ C 168.63, 157.50, 153.89, 153.61, 152.91, 139.96, 136.70, 134.36, 130.27, 127.93, 120.40, 118.96, 115.39, 110.25, 61.57, 16.22.
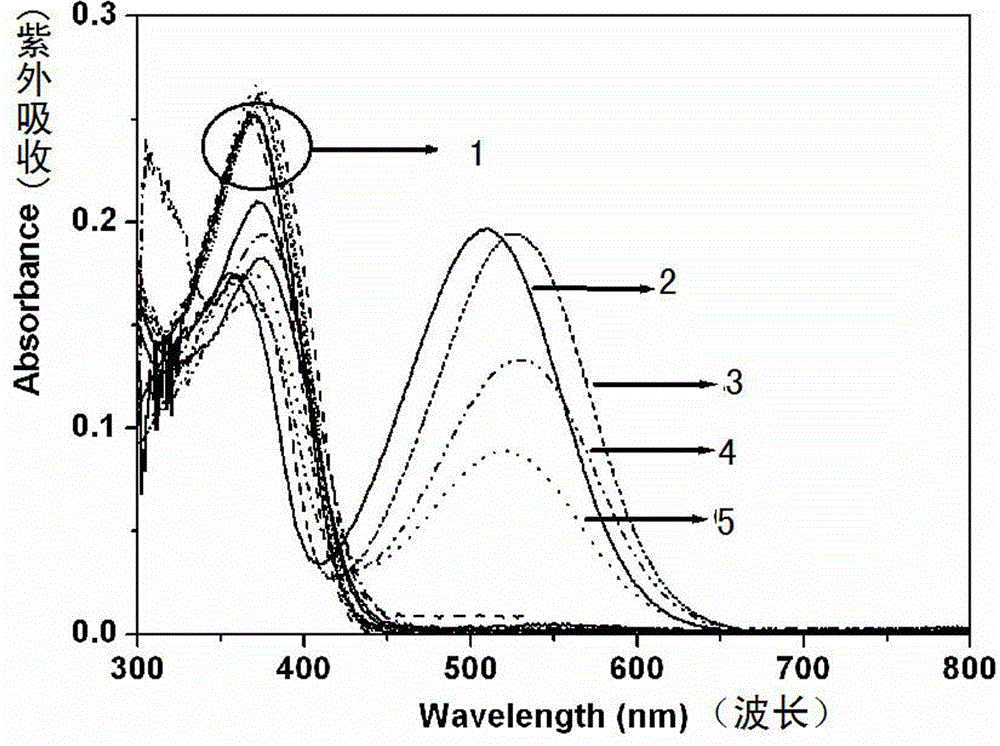
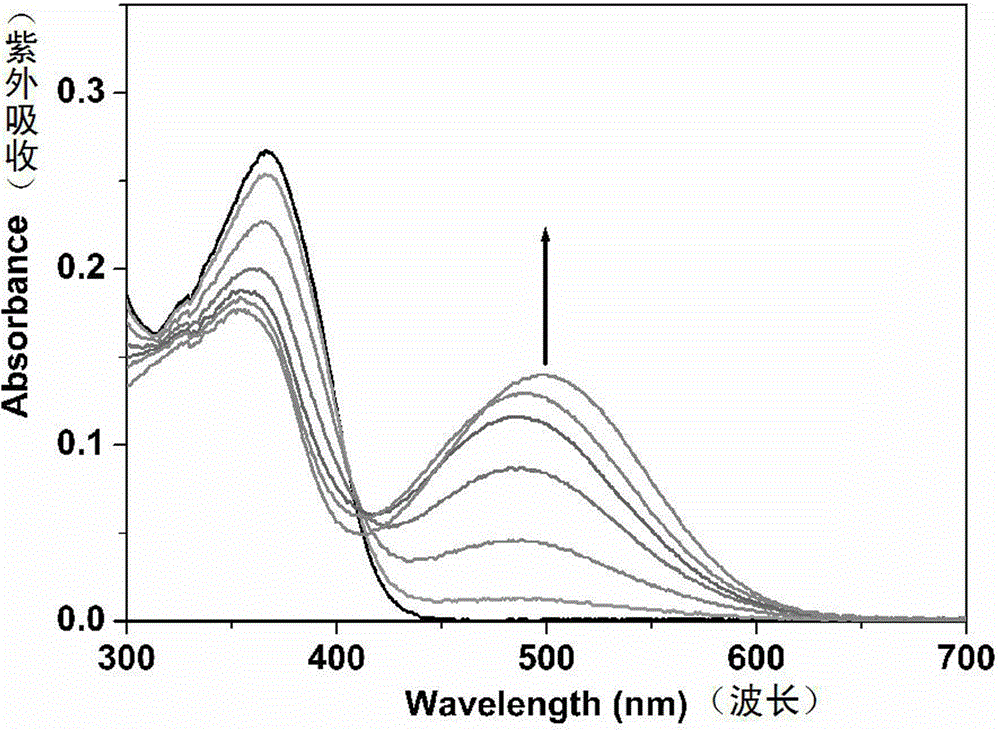
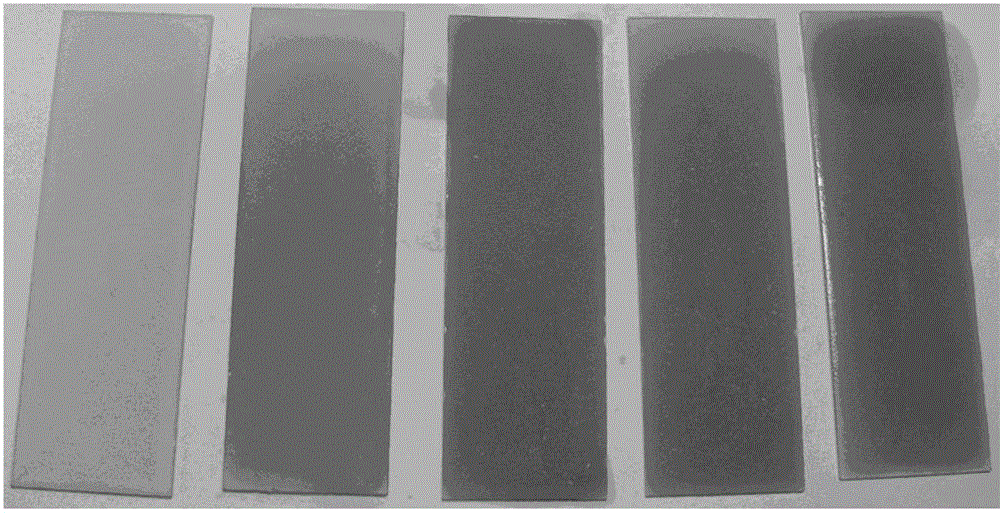
[0030] Probe molecules 1 and 2 (1.5 × 10 -5 M) add in corresponding solvent, measure ultraviolet-visible spectrum change, as figure 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com