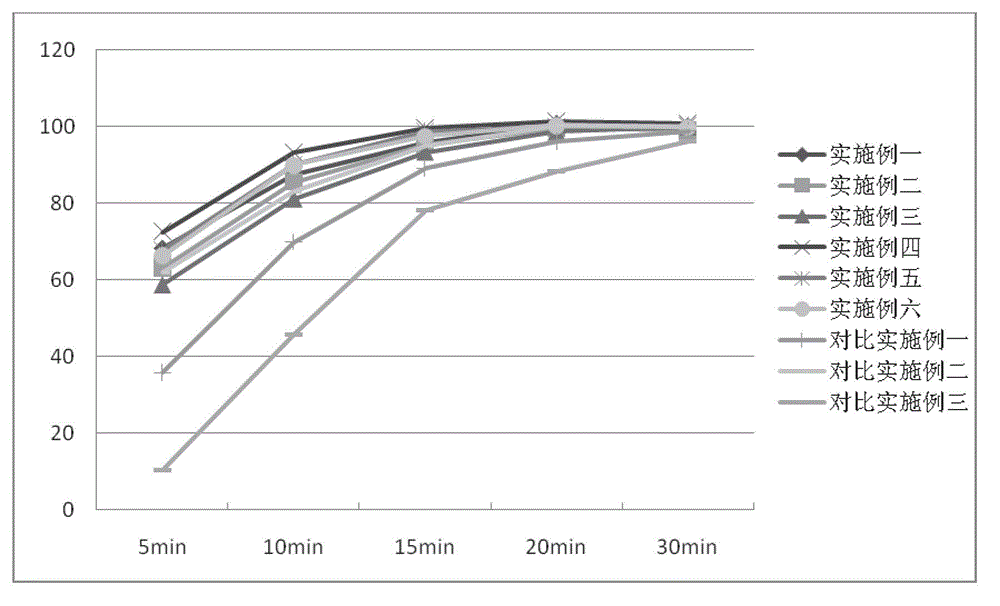
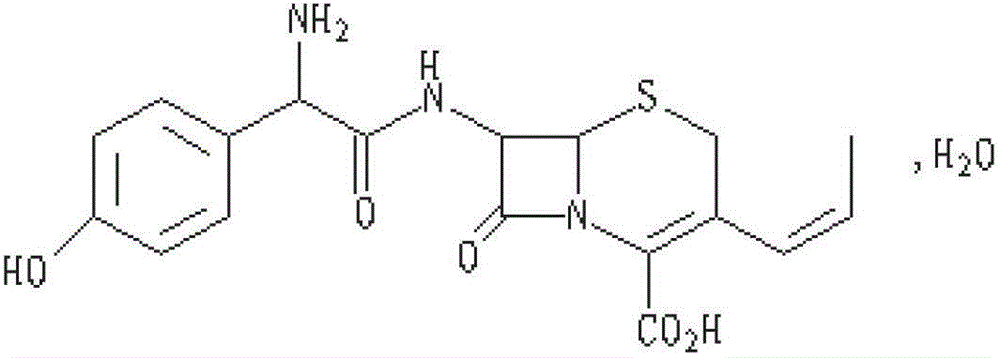
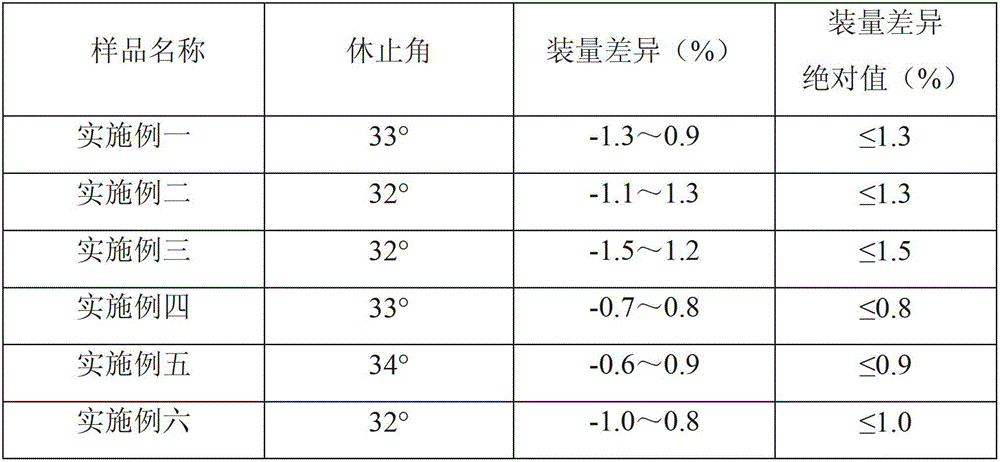
Cefprozil capsule and preparation method thereof
A technology for cefprozil and capsules, which is applied to the field of cefprozil capsules and its preparation, can solve the problems of not easy to disintegrate quickly and large particles, etc., and achieve the effects of stable drug effect, good fluidity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A cefprozil capsule comprises the following components in parts by weight:
[0045] Table 1. Embodiment a group of distribution ratio
[0046] components
Weight (parts)
cefprozil
125
8
[0047] A preparation method for cefprozil capsules, comprising the following steps (each component consumption is as described in Table 1):
[0048] 1) Sieving: Sieve cefprozil to control the particle size to 50μm≤d50≤120μm, 75μm≤d90≤200μm, and d100≤300μm;
[0049] 2) Mixing: add sodium starch glycolate and mix evenly;
[0050] 3) Intermediate detection;
[0051] 4) Filling: Cefprozil capsules are prepared.
Embodiment 2
[0053] A cefprozil capsule comprises the following components in parts by weight:
[0054] Table 2. Embodiment two groups distribution ratio
[0055] components
Weight (parts)
cefprozil
250
8
[0056] A preparation method of cefprozil capsules, comprising the following steps (each component consumption is as described in table 2):
[0057] 1) Sieving: Sieve cefprozil to control the particle size to 50μm≤d50≤120μm, 75μm≤d90≤200μm, and d100≤300μm;
[0058] 2) Mixing: add sodium starch glycolate and mix evenly;
[0059] 3) Intermediate detection;
[0060] 4) Filling: Cefprozil capsules are prepared.
Embodiment 3
[0062] A cefprozil capsule comprises the following components in parts by weight:
[0063] Table 3. Embodiment three groups distribution ratio
[0064] components
Weight (parts)
cefprozil
375
High expansion sodium carboxymethyl starch
8
[0065] A preparation method of cefprozil capsules, comprising the following steps (each component consumption is as described in table 3):
[0066] 1) Sieving: Sieve cefprozil to control the particle size to 50μm≤d50≤120μm, 75μm≤d90≤200μm, and d100≤300μm;
[0067] 2) Mixing: Add high-expansion sodium carboxymethyl starch and mix evenly;
[0068] 3) Intermediate detection;
[0069] 4) Filling: Cefprozil capsules are prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com