Application of germacrone in preparing HSV (Herpes Simplex Virus) resisting medicine
A technology of herpes simplex virus and gemmazone, which is applied in the field of medicine, can solve the problems of increasing the treatment of HSV infection, undetected, difficulties, etc., and achieve a good effect of anti-herpes simplex virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of gemmaquinone
[0026] The branches and leaves of Rhododendron xing'an were extracted twice with 80% ethanol under reflux, each time for 2 hours, the extracts were combined, concentrated under reduced pressure until there was no alcohol smell, centrifuged, and the supernatant was put on a macroporous resin chromatography column, and first washed with purified water Elute 3 column volumes, then elute 3 column volumes with 50% concentration ethanol, and then use 70% concentration ethanol to elute 5 column volumes, collect 70% concentration ethanol eluate, concentrate under reduced pressure, and crystallize to obtain Crude gemacerone, after dissolving the crude gemacerone in methanol, standing and recrystallizing, the pure gemacerone (purity>95.0%) can be obtained.
Embodiment 2
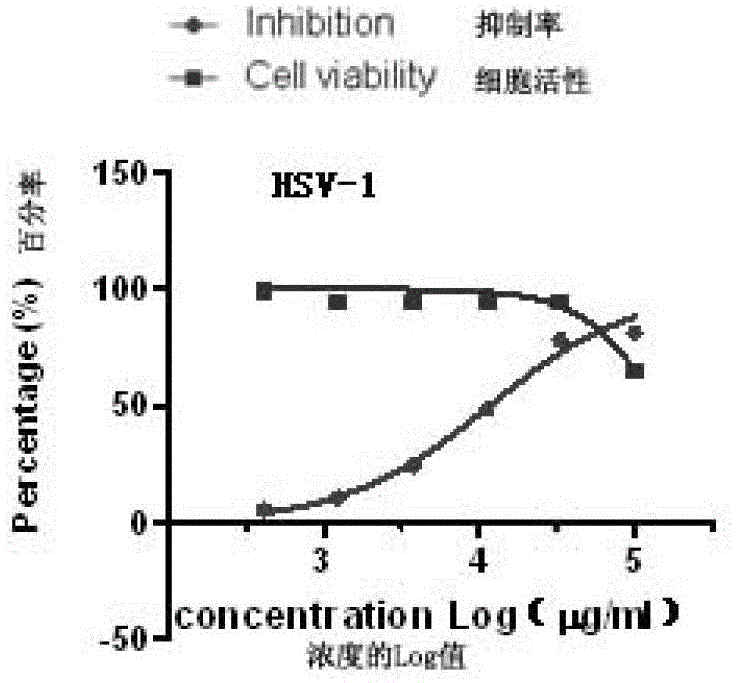
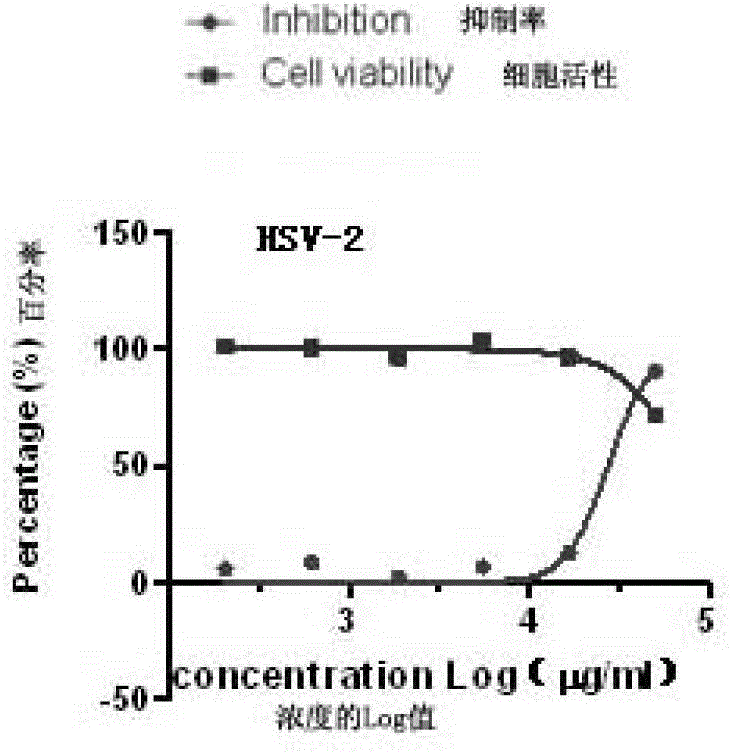
[0027] Embodiment 2: Anti-herpes simplex virus effect test of gemmaquinone in vitro
[0028] 1. Materials
[0029] 1.1 Strains: HSV-1, HSV-2.
[0030] 1.2 Cell model: monkey kidney cell line Vero.
[0031] Culture conditions: DMEM+10% fetal bovine serum, 37°C, 5% CO 2 .
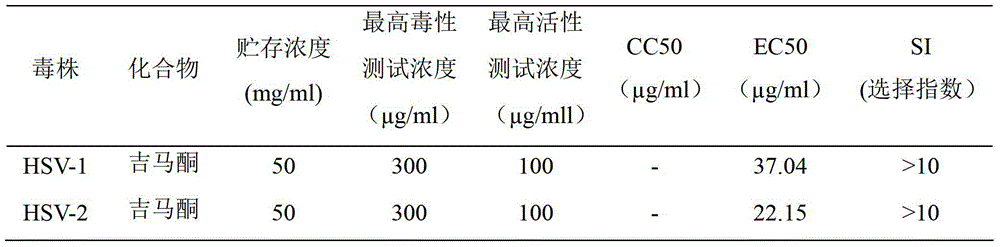
[0032] 1.3 Testing samples: See Table 1 for sample numbers and concentrations.
[0033] 2. Principles and methods
[0034] 2.1 Drug cytotoxicity detection
[0035] The alamarBlue (Invitrogen Company) kit was used to detect the toxic effect of drugs on cells.
[0036] Experimental principle: alamarBlue is a redox indicator that produces absorbance changes and fluorescent signals based on metabolic activity. AlamarBlue is easily soluble in water, and its oxidized form enters the cells and is reduced by mitochondrial enzymes to produce measurable fluorescence and color changes, which are used for quantitative analysis of cell viability and cell proliferation and in vitro cytotoxicity studies. This assay ...
Embodiment 3
[0054] Embodiment 3: Gemasolone is to the protective effect test of HSV-1 infection mouse
[0055] 1. Materials
[0056] 1.1 Strains The standard herpes simplex virus type I (HSV-1) SM44 strain was passaged in Vero cells.
[0057] 1.2 Animals Clean grade Kunming mice, half male and half male, mass 20±2g.
[0058] 1.3 The concentrations of gemmacone and acyclovir in samples are shown in Table 2.1 and 2.2.
[0059] 2. Test method
[0060] 48 Kunming mice were randomly divided into 6 groups: blank control group (0.9% normal saline); The equestrian high-dose group (40mg / Kg) and the positive drug control group (acyclovir 100mg / Kg) were given continuous intraperitoneal injection for 11 days. The mice were inoculated with the virus on the 4th day after administration (except the blank control group).
[0061] 2.1 Determination of body weight, life extension rate and mortality of virus-infected mice
[0062] The mice were weighed every two days, and the body weight changes and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com