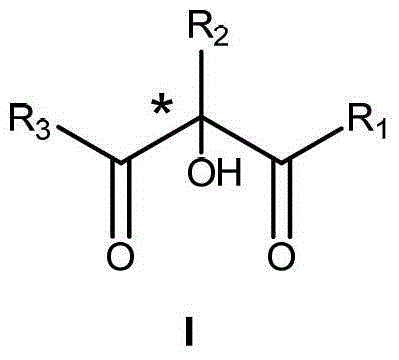
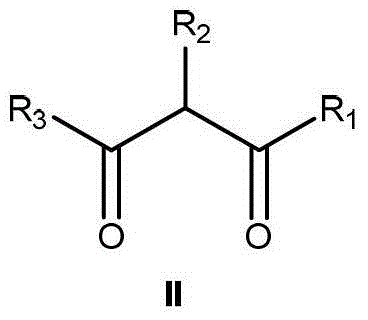
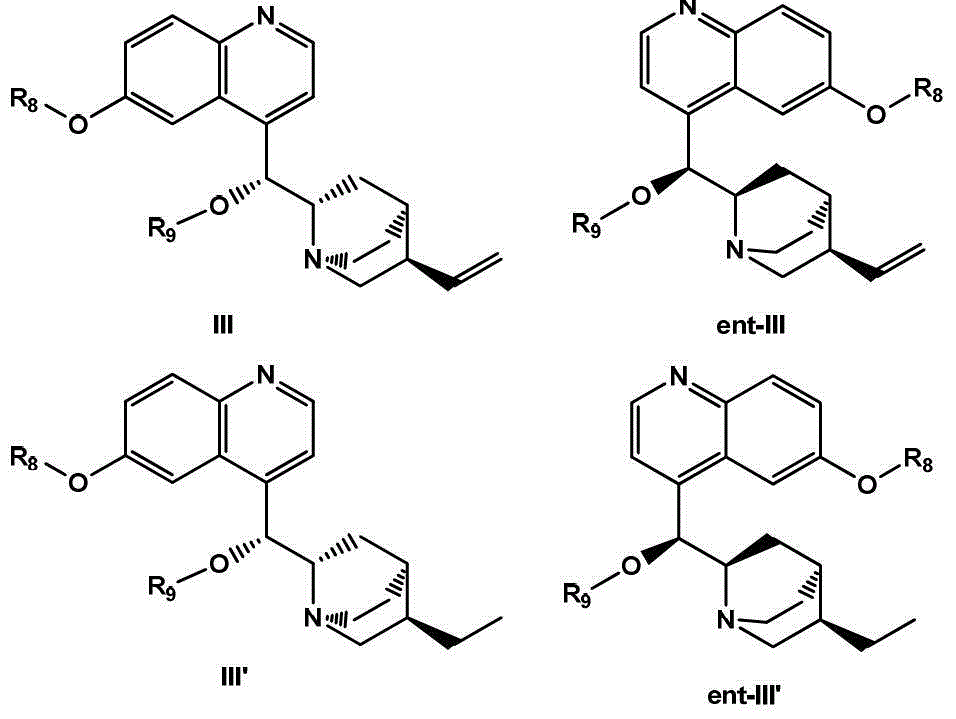
Preparation method of alpha-hydroxy-beta-dicarbonyl compound using cinchona alkaloid derivative as catalyst
A technology of dicarbonyl compounds and cinchona base, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of carboxylate esters, and can solve problems such as inapplicable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: prepare 9-oxygen-benzyl-6'-hydroxy cinchonidine (formula IIIa, wherein R 10 , R 11 for H).
[0086] Quinine (648 mg, 2.0 mmol) was dissolved in 20 ml of DMF, and 300 mg of 40% mass fraction of sodium hydride was added under nitrogen protection. After the mixture was stirred at room temperature for 1 hour, Bian bromide (423 mg, 2.5 mmol) was mixed with 2 ml of DMF, carefully added dropwise into the reactor, and stirred overnight at room temperature. After the raw materials were completely reacted, brine was added to quench the system, and the mixture was extracted with ethyl acetate. The combined organic extracts were dried over anhydrous sodium sulfate and evaporated in vacuo. The crude product was a yellow oil, which was directly used in the next reaction. Add 1.2 g of 40% mass fraction of sodium hydride to the dry DMF, and carefully add 2 ml of ethanethiol with a needle under the protection of nitrogen. After the reaction mixture was stirred for two...
Embodiment 2
[0087] Embodiment 2: 9-O-(3,5-tert-butyl)-benzyl-6'-hydroxy cinchonidine (formula IIIa, wherein R 10 is tert-butyl, R 11 for H)
[0088] With the preparation method of Example 1, silica gel column chromatography (elution gradient: CH 2 Cl 2 / MeOH / Et 3 N=100:5:1), a white solid was obtained with a final yield of 82%. [α] D 20 =-51.6(c0.25, CHCl 3 );mp:108-110℃; 1 H NMR (400MHz, CDCl 3 )δ8.69(d,J=4.4Hz,1H),8.02(d,J=9.0Hz,2H),7.47(d,J=3.9Hz,1H),7.37(s,1H),7.31(d, J=8.9Hz,1H),7.17(s,2H),5.74–5.52(m,2H),5.07–4.77(m,2H),4.47(s,3H),3.77(s,1H),3.25(t ,J=12.0Hz,1H),3.10(s,1H),2.90(d,J=7.3Hz,1H),2.69(d,J=10.5Hz,1H), 2.42(s,1H),2.13(s ,1H),2.00(s,1H),1.89(s,1H),1.65(s,1H),1.49(d,J=7.0Hz,1H),1.43–1.21(m,18H). 13 C NMR (101MHz, CDCl 3 )δ150.90,146.78,143.78,140.53,138.35,136.90,131.32,127.95,123.00,121.81,121.73,115.13,107.23,104.75,71.87,59.66,56.27,39.35,34.85,31.47,27.80,26.77.HRMS(ES + ) calcd for (C 34 h 44 N 2 o 2 +H + ):513.3405,found:513.3412.
Embodiment 3
[0089] Embodiment 3: prepare 9-O-(3-trifluoromethyl)-benzyl-6'-hydroxy cinchonidine (formula IIIa, wherein R 10 is trifluoromethyl, R 11 for H)
[0090] With the preparation method of Example 1, silica gel column chromatography (elution gradient: CH 2 Cl 2 / MeOH=15:1), a white solid was obtained with a final yield of 81%. [α] D 20 =-31.5(c0.25, CHCl 3 );mp:117-121℃; 1 H NMR (400MHz, CDCl 3 )δ8.71(d,J=4.2Hz,1H),8.12(s,1H),8.03(d,J=9.0Hz,1H),7.63(d,J=7.8Hz,2H),7.46(d, J=8.5Hz,3H),7.34(d,J=8.7Hz,1H),5.73(s,1H),5.68–5.53(m,1H),5.05–4.84(m,2H),4.49(dd,J =26.8,11.8Hz,2H),3.71(s,1H),3.39–3.12(m,2H),2.92(d,J=28.5Hz,1H),2.78(d,J=11.8Hz,1H),2.45 (s,1H),2.11(s,1H),1.93(s,2H),1.61(d,J=47.4Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ156.89,146.71,143.91,142.51,139.90,138.66,131.61,131.08,130.60,129.02,127.65,125.41,124.63,123.95,123.16,115.61,106.27,77.35,77.03,76.72,70.51,59.58,56.00,45.35,43.51 , 38.91, 27.57, 26.43. HRMS (ES + ) calcd for (C 27 h 27 f 3 N 2 o 2 +H + ):469.203...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com