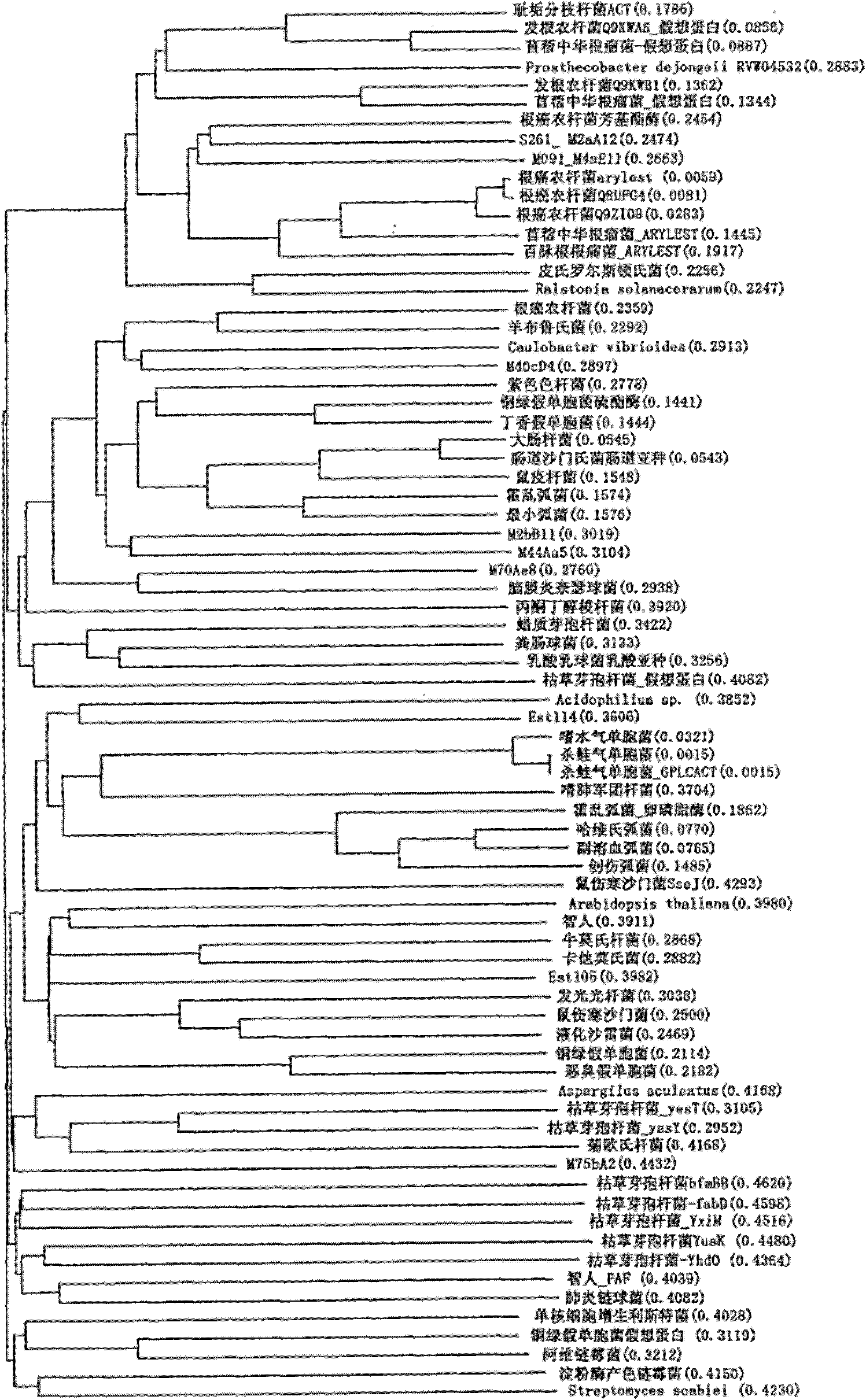
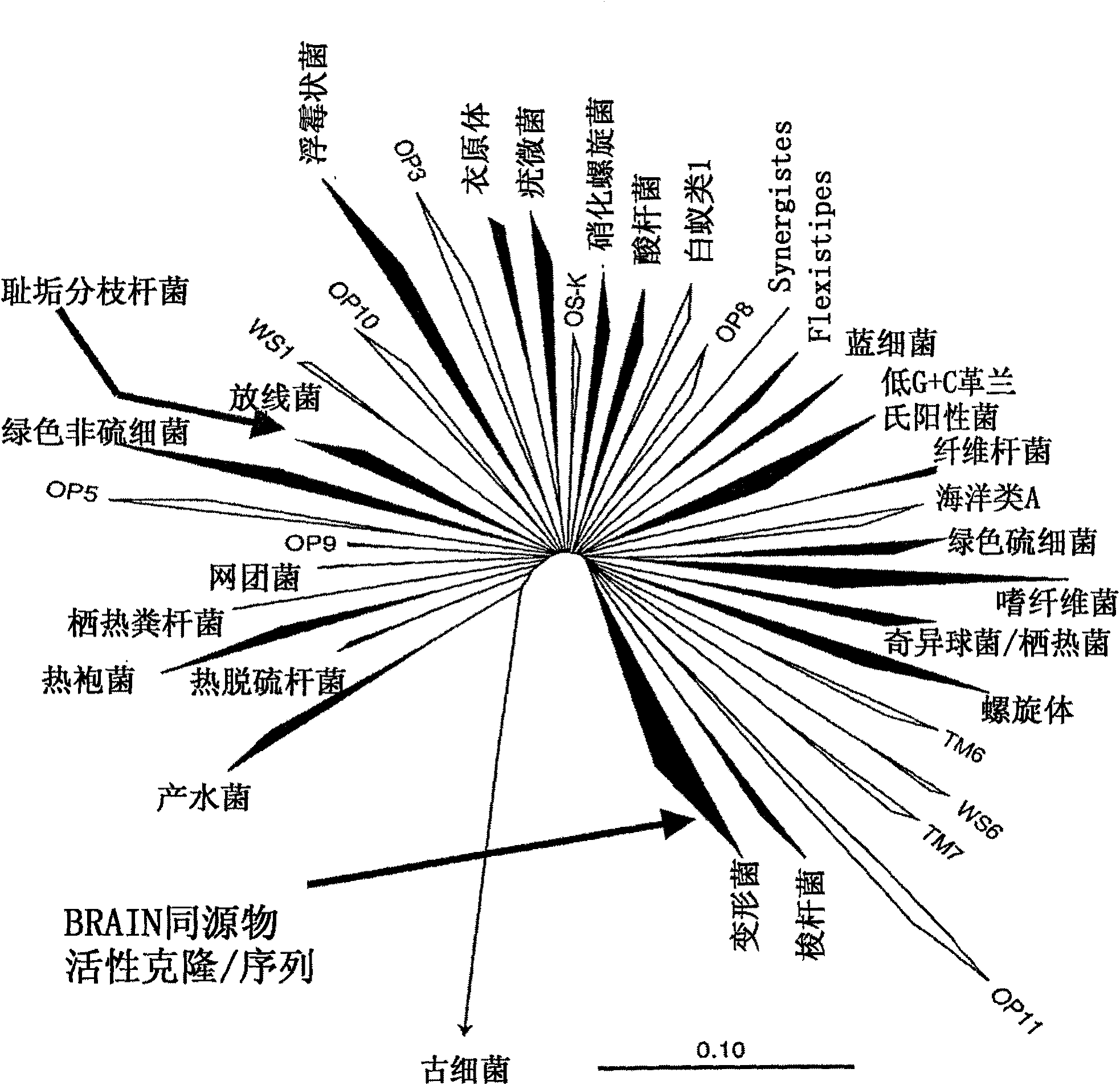
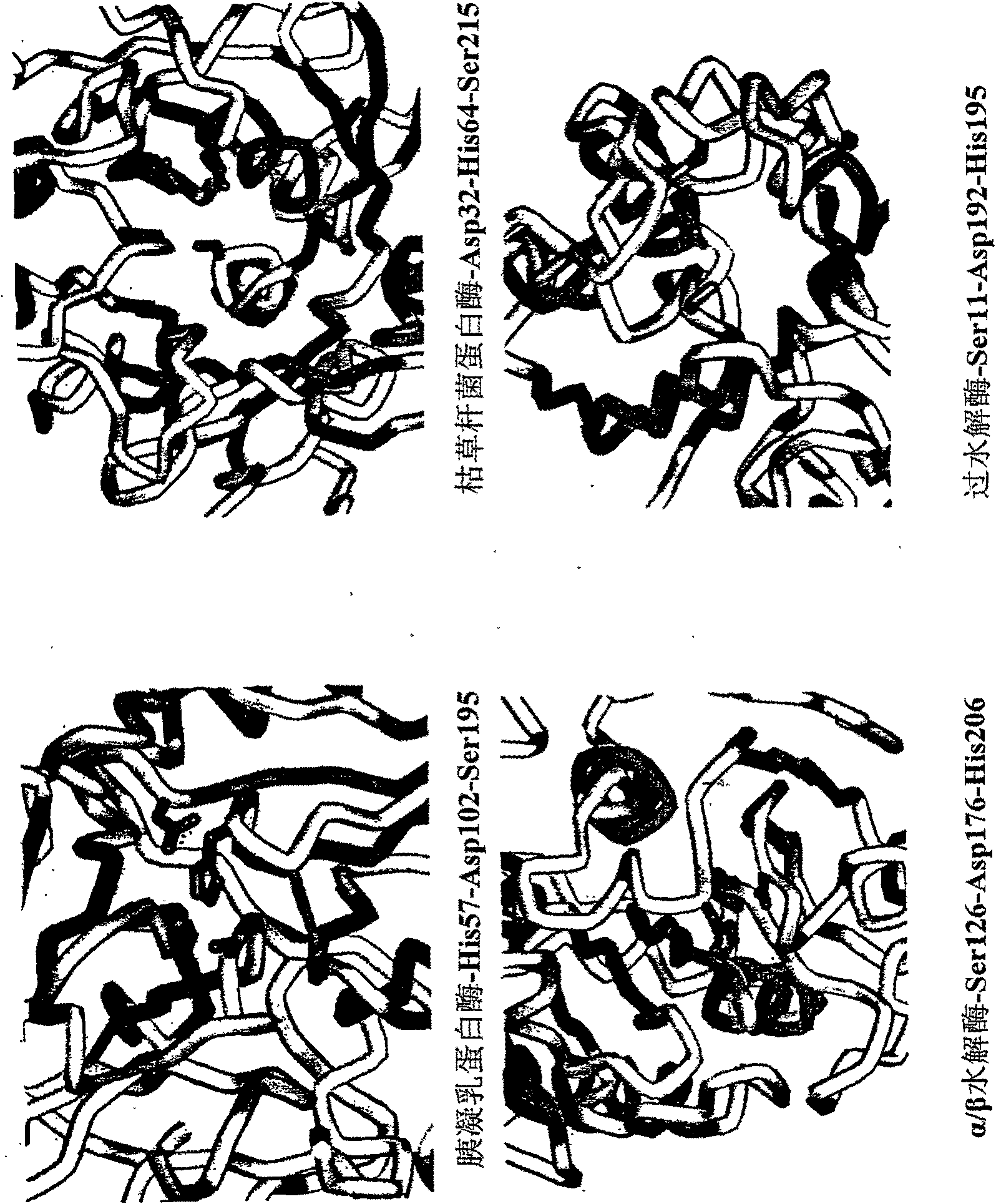
Perhydrolase enzyme
A technology of perhydrolase and perhydrolysis, which is applied in the direction of hydrolase, water supply device, detergent compounding agent, etc., which can solve the problems of difficult deinking and difficult removal of ink
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1338] Enzyme analysis
[1339] In this example, the methods used to assess enzyme purity and activity are described, which are used in the examples that follow and throughout the specification.
[1340] Enzyme activity assay (pNB assay)
[1341] The activity is determined by hydrolysis of p-nitrophenylbutyrate. The reaction mixture was prepared by adding 10 ul of 100 mM p-nitrophenylbutyrate in DMSO to 990 ml of 100 mM Tris-HCl buffer, pH 8.0, containing 0.1% triton X-100 . Background hydrolysis rates were measured at 410 nm prior to addition of enzyme. The reaction was initiated by adding 10 ul of enzyme to 990 ml of reaction mass and the change in absorbance at 410 nm was measured at room temperature (~23°C). Background corrected results are reported as ΔA 410 / min / ml or δA 410 / min / mg protein.
[1342] transesterification
[1343] Transesterification was determined by GC separation of the product in the buffered aqueous reaction. The reaction for the determination...
Embodiment 2
[1351] Determination of the ratio between peracid formation and acid formation
[1352] In this example, a method for determining the ratio of perhydrolysis to hydrolysis is described. Specifically, this example provides a method for determining the ratio between peracid formation (i.e., perhydrolysis) and acid formation (i.e., hydrolysis) in an aqueous system in the presence of peroxide In the case of , produced by the activity of an enzyme on an ester substrate.
[1353] A. Determination of the ratio of perhydrolysis to hydrolysis
[1354] Substrate preparation
[1355] Substrates were prepared as described here. Ethyl acetate (EtOAc) and other water-soluble esters were diluted in the desired buffer to achieve an ester concentration of 10 mM. Tributyrin and other water insoluble substrates were prepared by preparing substrate swatches. Polyester samples were cut from undyed polyester fabric (Polycotton, PCW 22) with a 5 / 8 inch punch and placed in 24-well microtiter pl...
Embodiment 3
[1390] Analysis of detergent-containing compositions
[1391] In this example, an assay system for screening for superior perhydrolase activity in detergents with specific substrates is provided. These assays include those that measure peracid degradation by perhydrolases as well as peracid synthesis activity of the enzymes.
[1392] Materials and Methods for Peracid Formation (PAF) and Peracid Degradation (PAD) Analysis
[1393] This section provides materials and methods for screening better perhydrolases with C9E2OAC ester substrates in Ariel.
[1394] Material:
[1395] Ariel Futur (P&G, Ariel "C") Free of Bleach, Fragrance and Enzyme
[1396] C9E2OAc (P&G)
[1397] 30% hydrogen peroxide (Sigma)
[1398] 32% peracetic acid ("peracid", PAA) (Sigma cat#) MW=76.05; 4.208M
[1399] Acetic acid, anhydrous MW=192.12
[1400] Potassium hydroxide MW=56.11
[1401] ABTS (Sigma cat# A1888) MW=548.68
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com