Actinomycete fibrinolytic enzyme and preparation method thereof
An actinomycetes and plasmin technology, applied in biochemical equipment and methods, microorganism-based methods, enzymes, etc., can solve the problems of reducing plasmin activity, decreasing fibrinolytic effect, and many separation and purification steps, etc. The effect of good fibrin dissolving performance, short enzyme production cycle and high enzyme production activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
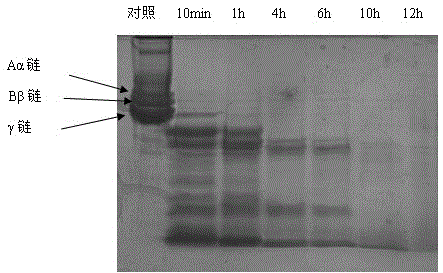
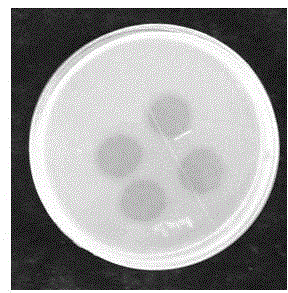
Image
Examples
Embodiment 1
[0025] 1. Strain: Actinomyces strain YY21, the preservation number is CGMCC N O .5816
[0026] 2. Medium and culture conditions:
[0027] (1) Incline medium: glucose 0.4-0.5%, yeast extract 0.3-0.6%, malt extract 0.5-1.5%, calcium carbonate 0.1-0.2%, agar 1.5%, pH6-7.
[0028] Slant culture conditions: culture at 28°C for 4-7 days.
[0029] (2) Seed medium: glucose 0.4-0.5%, yeast extract 0.3-0.6%, malt extract 0.5-1.5%, calcium carbonate 0.1-0.2%, adjust the pH to 6-7, and sterilize for later use.
[0030] Cultivation conditions: the rotation speed is 170-190r / min, 28°C shaking culture for 28-32h, as a liquid seed.
[0031] (3) Fermentation medium: millet flour 6-8%, glucose 2-4%, calcium carbonate 0.1-0.3%, sodium chloride 0.4-0.6%, peptone 0.6-0.7%, pH7.0-7.4, 5% inoculation quantity.
[0032] Culture conditions: 150-170r / min, 90-100h fermentation at 22°C.
Embodiment 2
[0034] 1. Incline cultivation is the same as in Example 1
[0035] 2, seed culture is the same as embodiment 1
[0036] 3, fermentation culture is the same as embodiment 1
[0037] 4. Separation and purification of actinomycete plasmin:
[0038] The liquid fermentation culture was centrifuged at 10000rpm / min, 4°C for 20 min, and the centrifuged supernatant was subjected to fractional salting out with 30%-60% W / V saturation of ammonium sulfate to obtain crude enzyme liquid.
[0039] a. Octyl-Sepharose FF hydrophobic interaction chromatography, the salt saturation of the sample is adjusted to 30%, and after loading the sample is eluted with a gradient of 30-0% W / V ammonium sulfate solution to collect fibrinolytic active components; and
[0040] b. Phenyl-Sepharose HP hydrophobic interaction chromatography, the salt saturation of the sample was adjusted to 15%, and after loading the sample was eluted with a gradient of 15-0% W / V ammonium sulfate solution, and fibr...
Embodiment 3
[0042] 1. Incline cultivation is the same as in Example 1
[0043] 2, seed culture is the same as embodiment 1
[0044] 3, fermentation culture is the same as embodiment 1
[0045] 4. The separation and purification method of actinomycetes plasmin is the same as in Example 2
[0046] 5. Determine the amino acid sequence of the isolated and purified plasmin : 经过Q-TOF2串联质谱测得其中五个肽段的氨基酸序列分别为:A-Q-S-V-P-Y-G-L-S-Q-L-K,其分子量为1289.6644;V-A-V-L-D-S-G-L-D-S-S-H-P-D-L-K,其分子量为1651.8243;N-S-L-E-S-T-A-T-N-L-G-N-P-A-G-A-T-Y-G-K,其分子量为1964.8844;h-p-n-w-T-n-t-n-v-r,其分子量为1237.5844;Y-P-S-V-L-A-V-G-A-V-D-N-G-V-S-N-K,其 The molecular weight is 1688.8043.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com