Edaravone injection solution and preparation method thereof
A technology of edaravone and injection, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as unsatisfactory anti-oxidation effects and threats to the lives of patients. Achieve good antioxidant properties, good therapeutic effect, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] An edaravone injection, containing vitamin C and glutathione in the edaravone injection. In the edaravone injection, the concentration of edaravone is 0.1%-0.2% (W / V), the concentration of vitamin C is 0.1%-0.5% (W / V), and the concentration of glutathione The concentration is 1.0%-10.0% (W / V).
[0042] The preparation method of this Edaravone injection comprises the steps:
[0043] a. Take the prescribed amount of Edaravone, add 1 / 2 to 1 / 3 of the prescribed amount of water for injection at a temperature of 90 to 100°C, and stir to completely dissolve the Edaravone to obtain an Edaravone solution;
[0044] b. Add the vitamin C and glutathione to the edaravone solution, stir evenly, add water for injection to 80% of the prescription amount, and then cool to 70-80°C to obtain a mixed solution;
[0045] c. Add medical activated carbon to the mixed solution, the amount of the medical activated carbon added is 0.3% (W / V), stir for 15 minutes, filter while hot to decarbonize...
Embodiment 2
[0060] This embodiment is a preferred scheme based on embodiment 1, and the quality of the raw materials used is the same as that of embodiment 1.
[0061] In the described Edaravone injection, the concentration of Edaravone is 0.15% (W / V), the concentration of vitamin C is 0.25% (W / V), and the concentration of glutathione is 5.0% (W / V). / V).
[0062] The specific consumption of each raw material is:
[0063] Edaravone: 750g, vitamin C: 1.25kg, glutathione: 25kg, dilute hydrochloric acid: appropriate amount, sodium hydroxide: appropriate amount, add water for injection to 500L.
[0064] Refer to Example 1 for the preparation method of this example.
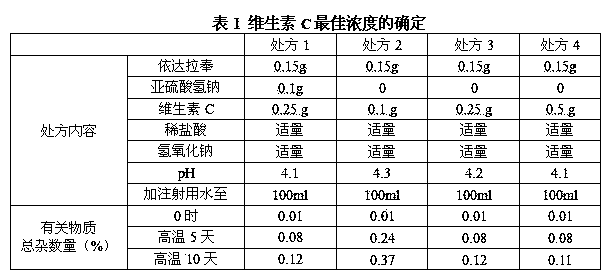
[0065] The optimum concentration of vitamin C dosage of the present embodiment is determined as shown in table 1:
[0066]
[0067] As can be seen from Table 1, the greater the concentration of vitamin C, the better the results of the influencing factors of Edaravone injection. Almost, it shows that the optimal dosage of vi...
Embodiment 3
[0084] This embodiment is a preferred scheme based on embodiment 1, and the quality of the raw materials used is the same as that of embodiment 1.
[0085] In the described Edaravone injection, the concentration of Edaravone is 0.1% (W / V), the concentration of vitamin C is 0.1% (W / V), and the concentration of glutathione is 1.0% (W / V). / V).
[0086] The specific consumption of each raw material is:
[0087] Edaravone: 2g, vitamin C: 2g, glutathione: 20g, dilute hydrochloric acid: appropriate amount, sodium hydroxide: appropriate amount, add water for injection to 2L.
[0088] Refer to Example 1 for the preparation method of this example.
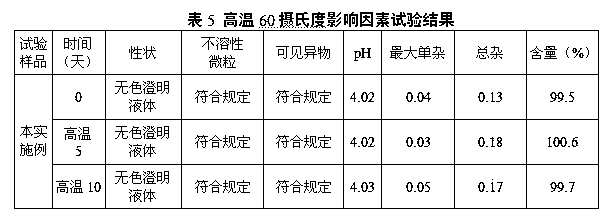
[0089] The sample of this embodiment is subjected to a high temperature 60 degree centigrade influence factor test. The detection method is referred to in Example 2. The detection data are as follows, see Table 5:
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com