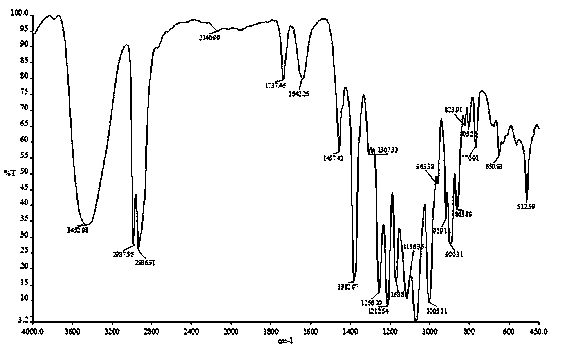
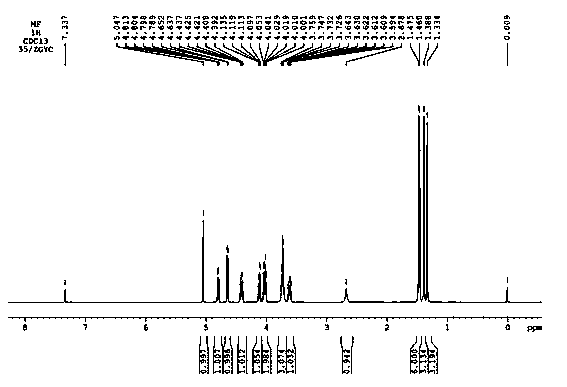
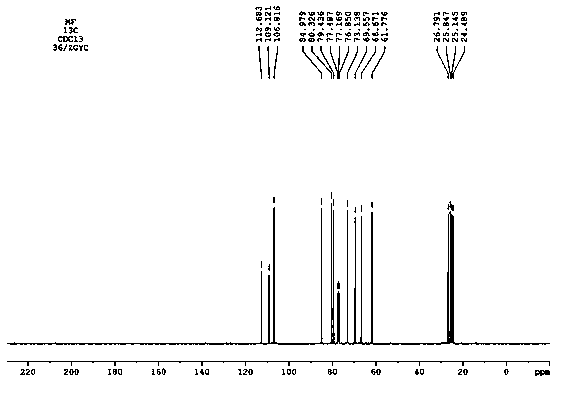
Tobacco humectant 1-O-hydroxyethyl-D-mannopyranose and preparation method thereof
A technology of mannose and humectant for tobacco, which is applied in the preparation of sugar derivatives, chemical instruments and methods, tobacco, etc., and can solve the problems of no moisture-proof effect, precipitation, low temperature and easy solidification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0031] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0032] Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 51 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.027mol, raised the temperature and reacted at 10°C 3h, TLC monitored the entire reaction process (V petroleum ether: V ethyl acetate = 3: 2), then slowly dropwise added 18mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filtered the precipitate, Concentration under reduced pressure gave 3.469 g of a colorless transparent viscous liquid (II), with a yield of 71.13%.
[0033] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
[0034] A...
Embodiment 2
[0036] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0037] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0038] Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannose furanose tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 60 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in an ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.03mol, raised the temperature and reacted at 15°C 4h, TLC monitored the entire reaction process (V petroleum ether: V ethyl acetate = 3: 2), and then slowly dropwise added 30mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filtered the precipitate, Concentration under reduced pressure gave 4.338 g of a colorless transparent viscous liquid (II), with a yield of 88.95%.
[0039] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
...
Embodiment 3
[0042] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0043] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0044] Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl tert-butyl ester (I) into a 250 mL three-necked flask, add 96 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in an ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.033mol, raised the temperature and reacted at 25°C 5h, TLC monitored the entire reaction process (V petroleum ether: V ethyl acetate = 3: 2), and then slowly dropwise added 40mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filtered the precipitate, Concentration under reduced pressure gave 4.2 g of a colorless transparent viscous liquid (II), with a yield of 86.12%.
[0045] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
[0046...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com