Compound intravenous anesthetic
An anesthetic and intravenous technology, applied in the field of medicine, can solve the problems of anesthesia risk, increased adverse reactions, and limited clinical application range, and achieve the effect of eliminating adverse reactions, low incidence of adverse reactions, and mild degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
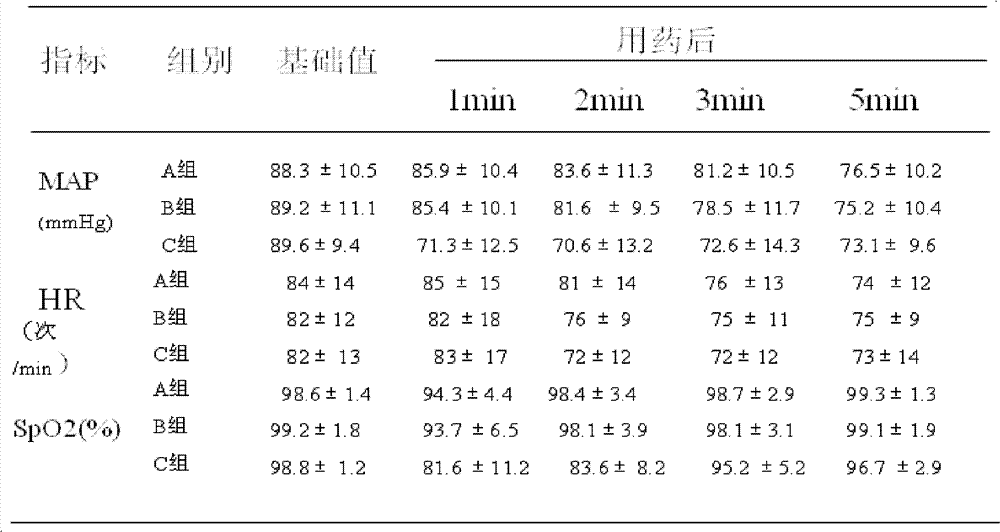
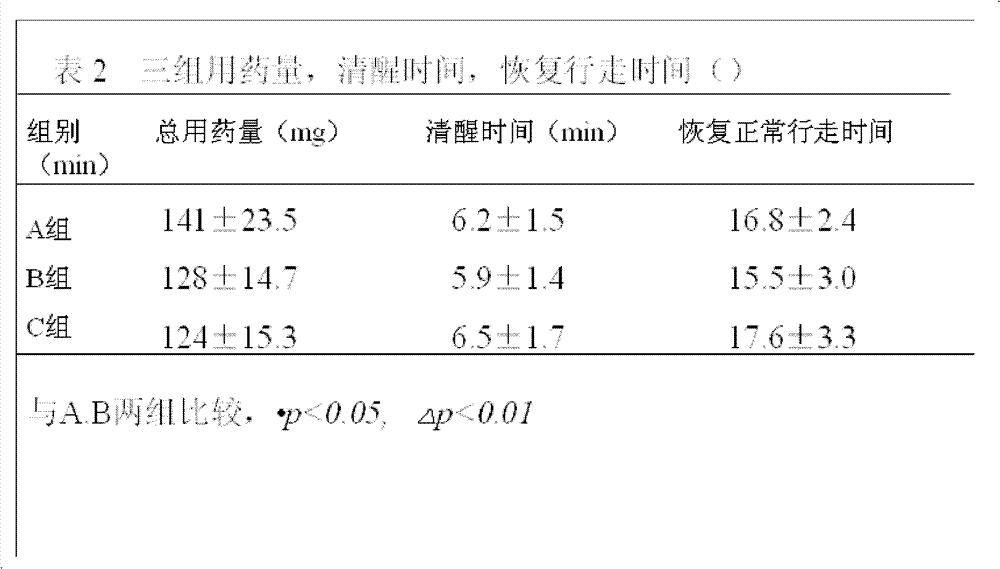
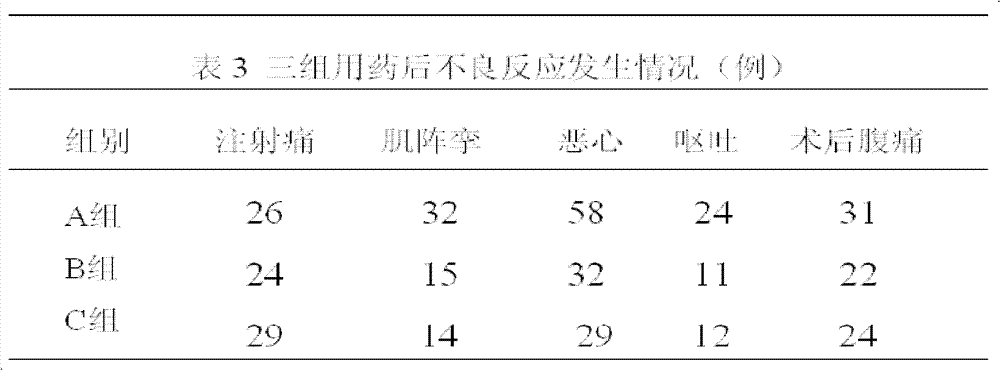
[0017] 1.1 Case selection: 600 cases of early pregnancy patients in the obstetric outpatient department, aged 20-35 years old, weight 40-75kg; ASA score I, no history of major organ diseases, no history of drug allergy. Routine preoperative examination: blood analysis, coagulation function, EKG, no food and drink before surgery for 8 hours. The cases were randomly divided into different ratio comparison groups of propofene (200mg / 20ml)-etomidate (20mg / 10ml) fat emulsion mixture. 200 cases per group.
[0018] Group A, dose ratio (mass ratio) 2.5:1; Group B, dose ratio (mass ratio) 4:1; Group C, dose ratio (mass ratio) 5.5:1.
[0019] 1.2 Drug equivalent dose: The equivalent dose ratio of propofol: etomidate is about 8.3:1; the equivalent dose of propofol in the mixture of group A is 14.4 mg / ml; the equivalent dose of propofol in the mixture of group B The concentration is 13.7mg / ml; the equivalent dose of propofol in group C mixture is 13.1mg / ml;
[0020] 1.3 Anesthesia: After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com