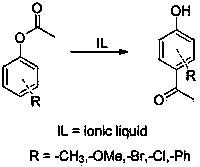Method for preparing p-hydroxy phenyl ethyl ketone compound through rearrangement reaction under catalysis of acidic ionic liquid
An acidic ionic liquid and a technology for p-hydroxyacetophenone, which is applied in the field of catalytic preparation of p-hydroxyacetophenone compounds, can solve the problems of serious environmental pollution, high corrosiveness, poor catalyst stability, etc., and achieves high reaction selectivity, selective The effect of specificity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
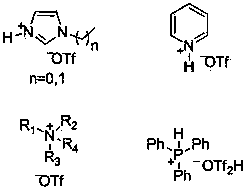
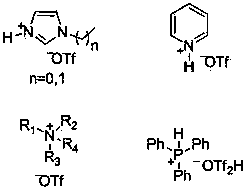
[0025] Experimental method: Add 5mmol of phenyl acetate into a 25mL one-necked flask, add 5mmol of N - Ethylimidazole trifluoromethanesulfonate ionic liquid, the reaction was stopped after stirring at 60° C. for 12 hours. Add 2 mL of water to dilute, extract three times with 2 mL of ethyl acetate, and wash the ethyl acetate layer with anhydrous MgSO 4 Dry and distill under reduced pressure to obtain p-hydroxyacetophenone with a yield of 18%.
Embodiment 2
[0027] Experimental method: Add 5 mmol of phenyl acetate into a 25 mL one-necked flask, add 5 mmol of 2-picoline trifluoromethanesulfonate ionic liquid, stir and react at 60° C. for 12 h, then stop the reaction. Add 2 mL of water to dilute, extract three times with 2 mL of ethyl acetate, and wash the ethyl acetate layer with anhydrous MgSO 4 Dry and distill under reduced pressure to obtain p-hydroxyacetophenone with a yield of 16%.
Embodiment 3
[0029] Experimental method: Add 5 mmol of phenyl acetate into a 25 mL single-necked flask, add 5 mmol of tri-n-octylamine trifluoromethanesulfonate ionic liquid, stir and react at 60° C. for 12 h, then stop the reaction. Add 2 mL of water to dilute, extract three times with 2 mL of ethyl acetate, add 2 mL of water to dilute, extract three times with 2 mL of ethyl acetate, wash the ethyl acetate layer with anhydrous MgSO 4 Dry and distill under reduced pressure to obtain p-hydroxyacetophenone with a yield of 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com