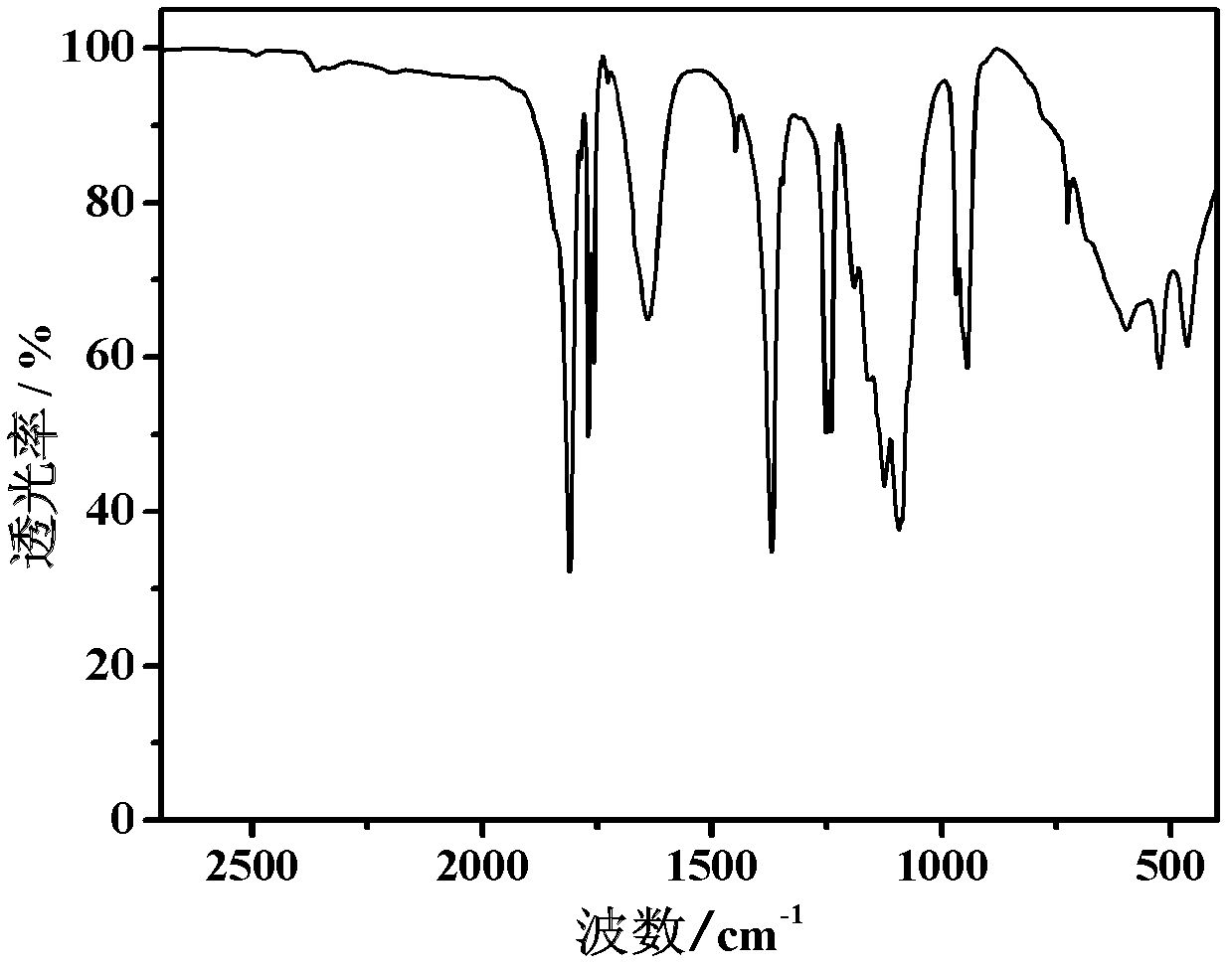
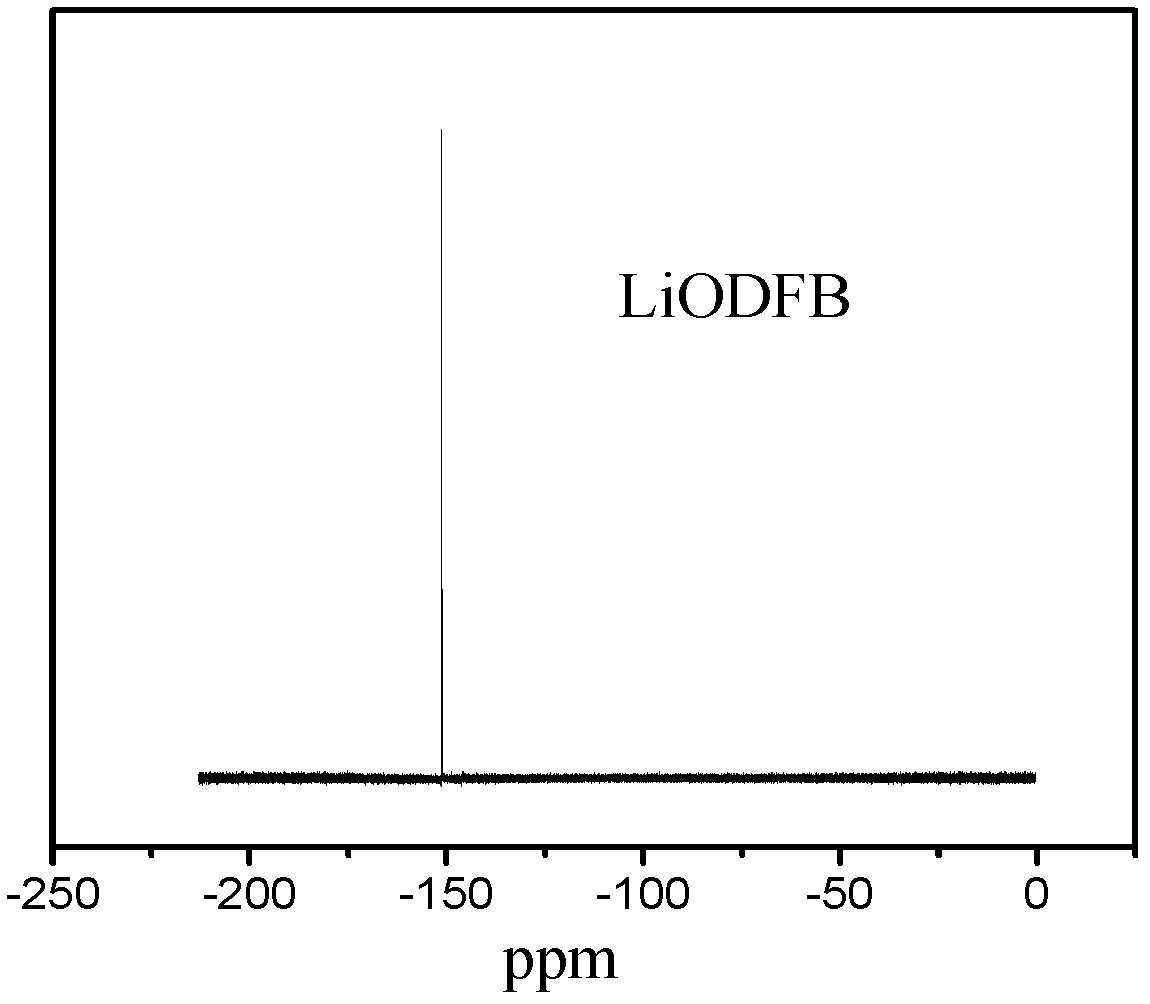
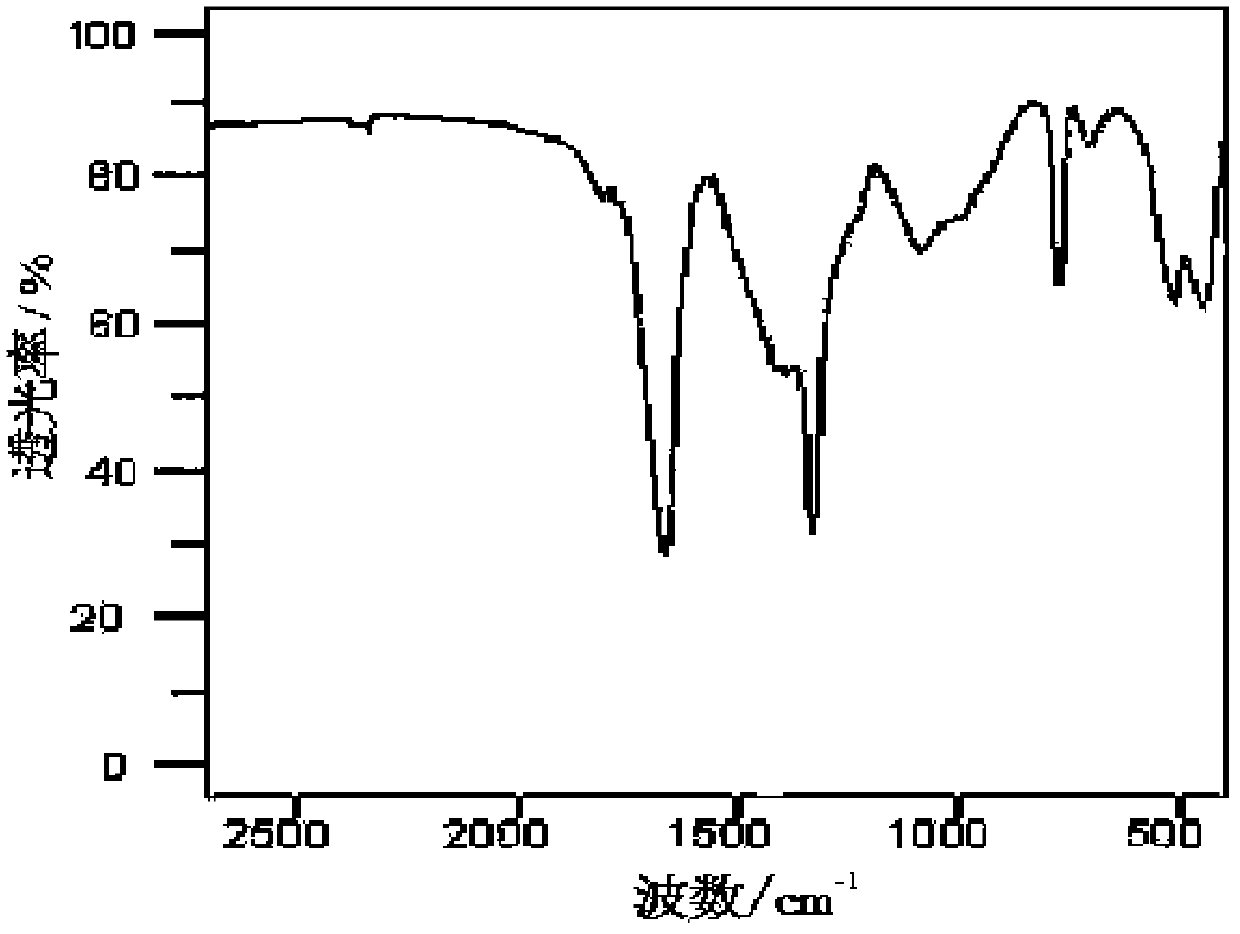
Co-production method for lithium oxalyldifluoroborate and lithium tetrafluoroborate
A technology of lithium oxalate difluoroborate and lithium tetrafluoroborate is applied in chemical instruments and methods, boron halide compounds, compounds containing elements of Group 3/13 of the periodic table, etc. The problem of unevenness and high product yield can achieve the effect of low production cost, high product purity and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The coproduction method of lithium oxalate difluoroborate and lithium tetrafluoroborate, the steps are:
[0015] (1) Add 10g of lithium oxalate and 25mL of anhydrous diethyl ether dried at 120°C for 4 hours into a dry reactor equipped with a stirrer and stir evenly, then add 24.6mL of BF 3 ·O(C 2 h 5 ) 2 (BF 3 The concentration is 45%~47%) solution, heat reflux and stir, to ensure that the raw materials are fully mixed. React at 80°C for 6h, filter and separate the solid-liquid mixture after the reaction, and then dry the separated solid at 50°C to 170°C under normal pressure for 6h, add a desiccant to the drying oven, and continue drying for 6h, Get LiODFB and LiBF 4 mixture of crude products.
[0016] (2) The crude product mixture was dissolved in 25 mL of diethyl carbonate in a reactor with a stirrer, and stirred and refluxed at 110° C. for 3 h. After the solution was filtered, it was evaporated at 110°C, and after reaching a saturated solution, it was crystal...
Embodiment 2
[0021] The coproduction method of lithium oxalate difluoroborate and lithium tetrafluoroborate, the steps are:
[0022] (1) will BF 3 Gas passes through H 2 o 2 0.1%~0.2%,H 2 O 2%~10% and H 2 SO 4 90% to 98% of the absorption liquid is washed and passed into the buffer bottle. Weigh 9g Li 2 C 2 o 4 Add 30mL of pretreated fresh anhydrous acetonitrile to the 100mL three-neck flask containing the magnetron, and slowly pass the purified BF from the buffer bottle into it under stirring conditions. 3 , heated up to 80°C, condensed and refluxed for 14h, and filtered with suction. The filtrate was concentrated until anhydrous acetonitrile was distilled off, the heating was stopped, the remaining liquid was concentrated, cooled and crystallized, the obtained solid was suction filtered and dried at 150°C and a vacuum of 0.08 MPa for 24 hours to obtain a white solid, which was detected as LiODFB and LiBF 4 Mixture of crude products.
[0023] (2) The mixture of crude products w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com