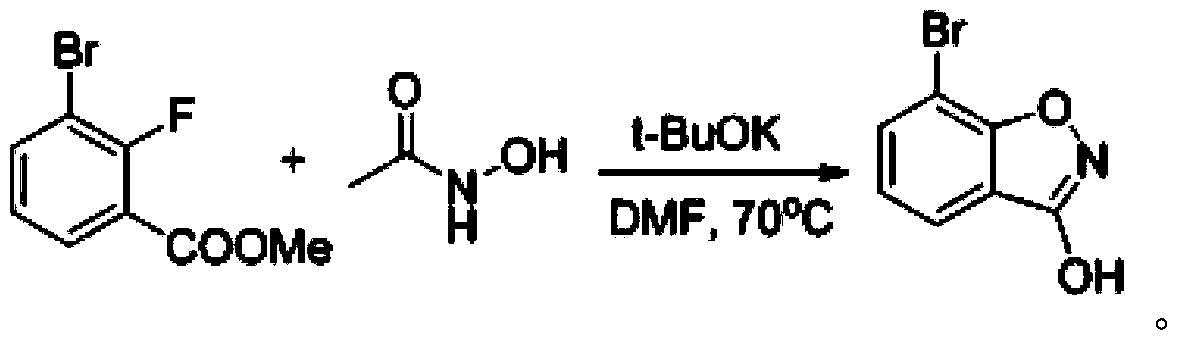3-chloro-7(5)-bromo-benzo-isoxazole compounding method
A technology of benzisoxazolone and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, expensive raw materials, cumbersome reaction steps, etc., and achieve fewer reaction steps, simple post-treatment, and high overall yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
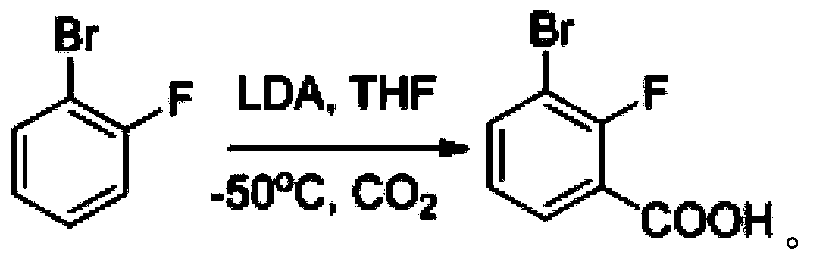
[0045] One, the synthesis of 3-chloro-7-bromobenzisoxazole
[0046] (1) Synthesis of 2-fluoro-3-bromobenzoic acid
[0047] Dissolve 13.5g of o-fluorobromobenzene in 140mL of anhydrous tetrahydrofuran and cool to -70°C, add 105mL of LDA with a concentration of 1.0mol / L dropwise under the protection of nitrogen, and the LDA is added dropwise within 30min. After the addition, continue to stir for 30 minutes at a constant temperature of -70°C, then pour the reaction solution into a reaction container filled with 200g of dry ice, stir until no bubbles are generated, spin dry, add 200mL of water to the reaction container to dilute to obtain a dilution , adjust the pH of the diluent to 5, filter, wash the filter cake with ice water, and dry it in vacuum at 50°C to obtain 16.2 g of white solid, namely 2-fluoro-3-bromobenzoic acid, with a yield of 95.9%.
[0048] LC-MS [ESI, m / z]: [M+1] + =218.9 / 220.8(1 / 1).
[0049] 1 H NMR (400MHz, DMSO-d 6 )δ13.60(s,1H),7.94(ddd,J=8.0,6.4,1.7Hz,...
Embodiment 2
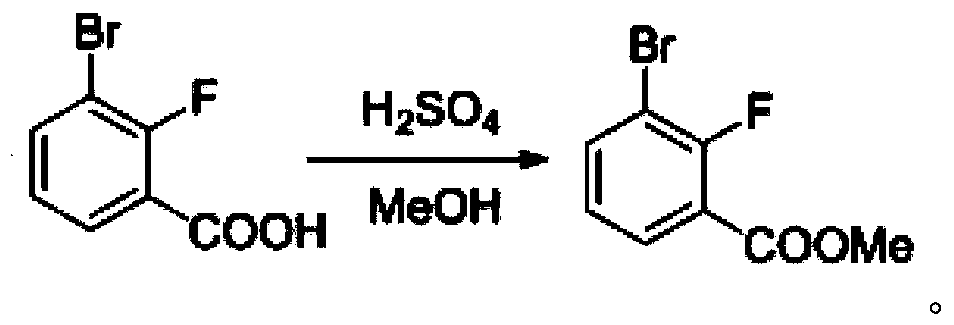
[0075] One, the synthesis of 3-chloro-7-bromobenzisoxazole
[0076] (1) Synthesis of 2-fluoro-3-bromobenzoic acid
[0077] Dissolve 14g of o-fluorobromobenzene in 145mL of anhydrous tetrahydrofuran and cool to -67°C, add 102mL of LDA with a concentration of 1.1mol / L dropwise under nitrogen protection. After completion, continue to stir for 35 minutes at a constant temperature of -67°C, then pour the reaction solution into a reaction container filled with 205 g of dry ice, stir until no bubbles are generated, spin dry, and add 205 mL of water to the reaction container to dilute to obtain a dilution. Adjust the pH of the diluent to 4.5, filter, wash the filter cake with ice water, and dry it under vacuum at 55°C to obtain 16.95 g of white solid, namely 2-fluoro-3-bromobenzoic acid, with a yield of 96.8%.
[0078] LC-MS [ESI, m / z]: [M+1] + =218.9 / 220.8(1 / 1).
[0079] 1 H NMR (400MHz, DMSO-d 6 )δ13.60(s,1H),7.94(ddd,J=8.0,6.4,1.7Hz,1H),7.86(ddd,J=8.3,6.8,1.7Hz,1H),7.26(t,J=8....
Embodiment 3
[0105] One, the synthesis of 3-chloro-7-bromobenzisoxazole
[0106] (1) Synthesis of 2-fluoro-3-bromobenzoic acid
[0107] Dissolve 14.5g of o-fluorobromobenzene in 150mL of anhydrous tetrahydrofuran and cool to -65~-70°C, add 105mL of LDA with a concentration of 1.0mol / L dropwise under the protection of nitrogen, the LDA is added dropwise within 30min, and the Stir, after the dropwise addition, continue to stir for 40min at a constant temperature of -65°C, then pour the reaction solution into a reaction vessel with 210g of dry ice, stir until no bubbles are generated, spin dry, add 210mL of water to the reaction vessel to dilute The diluted solution was obtained, and the pH of the diluted solution was adjusted to 4, then filtered, the filter cake was washed with ice water, and dried under vacuum at 60°C to obtain 17.32 g of white solid, namely 2-fluoro-3-bromobenzoic acid, with a yield of 95.4%.
[0108] LC-MS [ESI, m / z]: [M+1] + =218.9 / 220.8(1 / 1).
[0109] 1 H NMR (400MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com