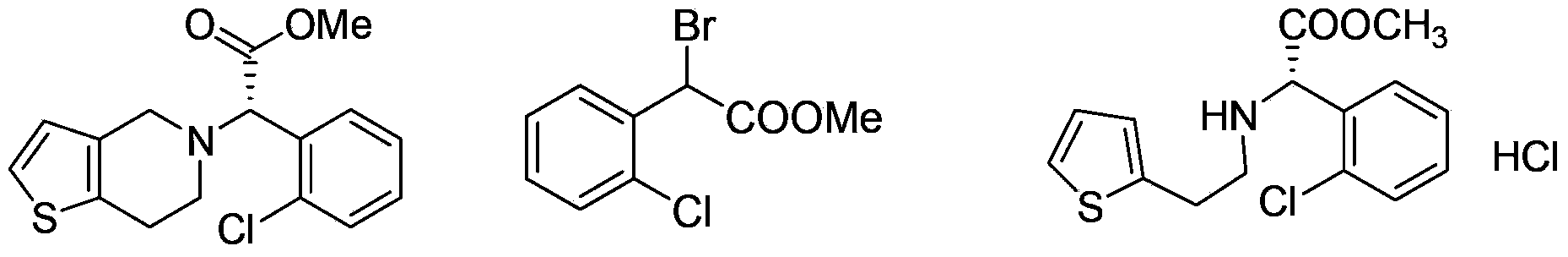
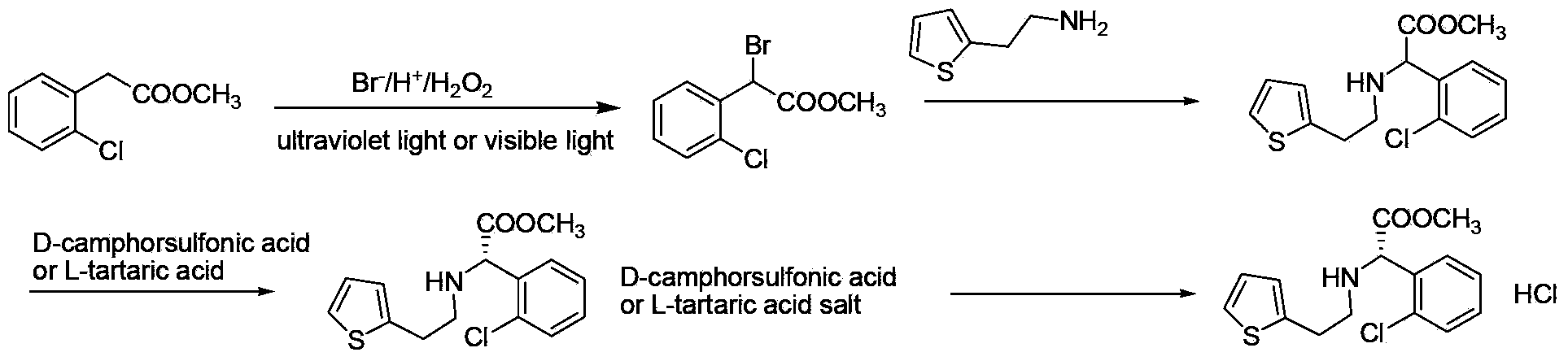
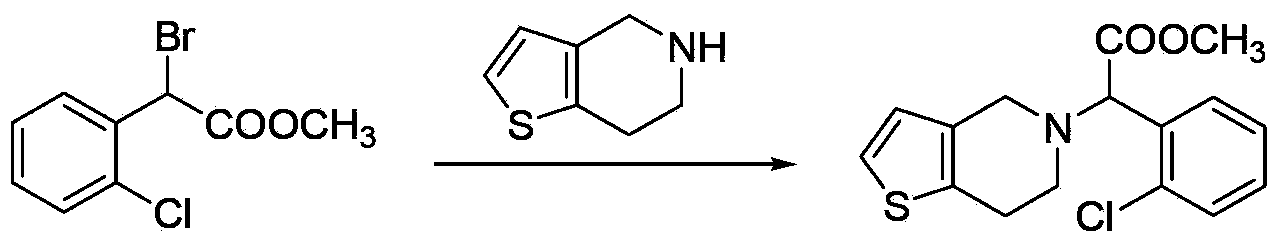
Preparation methods for clopidogrel and its intermediates--methyl alpha-bromo-2-chloro-phenylacetate and thiophene ethylamino-substituted methyl acetate
A technology of thienylethylamine and methyl acetate, which is applied in the field of preparation of medicine and its intermediates, can solve the problems of high cost and complicated production steps, achieve high utilization rate, and avoid the use and reaction of highly toxic bromination reagents The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] α-Bromo-o-chlorophenylacetic acid methyl ester
[0052] Add 2.2g of methyl o-chlorophenylacetate into a 100ml round bottom flask, and add 8ml of dichloromethane to dissolve it completely. Add potassium bromide 1.7g, add dropwise 1.4g50%H 2 SO 4 , put a 25W fluorescent lamp (color temperature 6400K) at a distance of 20cm from the round bottom flask reactor to irradiate the reaction, and slowly add 3.0g16%H at room temperature 2 o 2 , the reaction solution turns brownish red. After about 8 hours, the color of the reaction solution gradually faded, and the reaction was detected by HPLC, and the reaction of the raw materials was basically complete. Stop stirring and let stand to separate layers. The organic phase was sequentially washed with 5ml 5% NaHCO 3 solution and washed with 5ml of water. After concentrating under reduced pressure and reclaiming the organic solvent, 3.0 g of brown-yellow oil was obtained, and the purity of the product detected by HPLC was 99.0%...
Embodiment 2
[0054] α-Bromo-o-chlorophenylacetic acid methyl ester
[0055] Add 2.2g of methyl o-chlorophenylacetate into a 100ml round bottom flask, and add 8ml of dichloromethane to dissolve it completely. Add 1.8g of 50%H successively under stirring 2 SO 4 and potassium bromide 2.1g, then slowly drop 3.8g16%H 2 o 2 , and at the same time place a 25W fluorescent lamp (color temperature 6400K) at room temperature at a distance of 20cm from the round bottom flask to irradiate the reaction. After about 8 hours, the color of the reaction solution gradually faded, and the reaction was detected by HPLC, and the reaction of the raw materials was basically complete. Stop stirring and let stand to separate layers. The organic phase was sequentially washed with 5ml 5% NaHCO 3 solution and washed with 5ml of water. After concentrating under reduced pressure to recover the organic solvent, 3.0 g of brown oil was obtained, and the purity of the product was 99.3% by HPLC (the chromatographic co...
Embodiment 3
[0057] α-Bromo-o-chlorophenylacetic acid methyl ester
[0058] Add 2.2g of methyl o-chlorophenylacetate into a 100ml round bottom flask, and add 8ml of dichloromethane to dissolve it completely. Add 3.9g30% hydrobromic acid aqueous solution, then add 1.5g16%H 2 o 2 , the reaction solution turns brownish red. At room temperature, place a 25W fluorescent lamp (color temperature 6400K) at a distance of 20cm from the round bottom flask to irradiate the reaction, and slowly add another batch of 1.5g16%H 2 o 2 . After about 8 hours, the color of the reaction solution gradually faded, and the reaction was detected by HPLC, and the reaction of the raw materials was complete. Stop stirring and let stand to separate layers. The organic phase was sequentially washed with 5ml 5% NaHCO 3 solution and washed with 5ml of water. After concentrating under reduced pressure to recover the organic solvent, 3.0 g of brown oil was obtained. The purity of the product detected by HPLC was 99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com