Preparation methods for mirabegron and intermediate thereof
A technology of intermediates and compounds, which is applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, complicated operation, and high cost, and achieve the effects of mild reaction conditions, simple operation, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
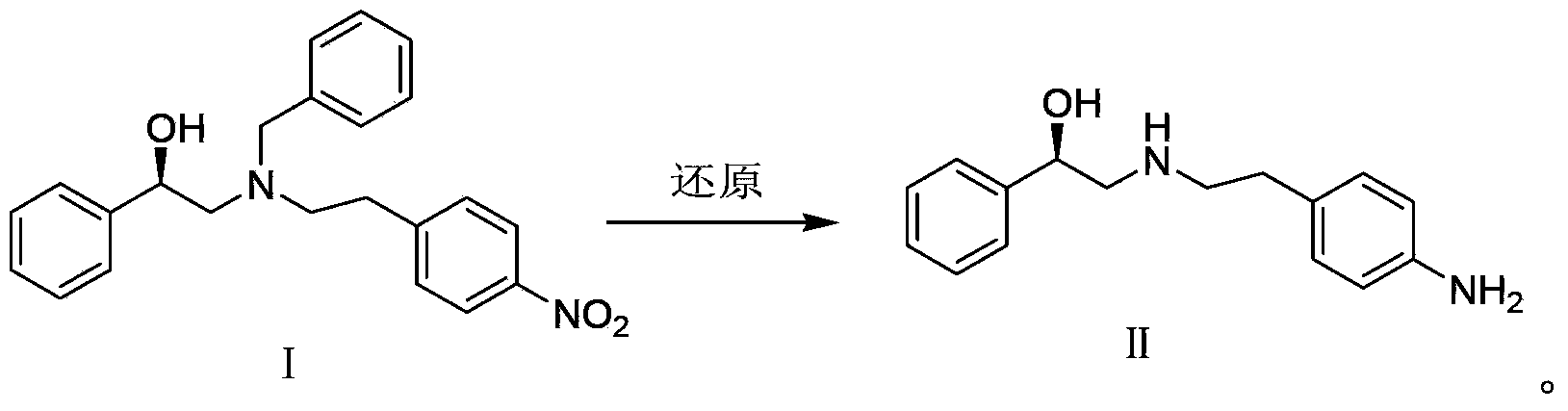
[0021] The preparation of embodiment 1 (R)-2-{N-benzyl-[2-(4-nitrophenyl) ethyl] amino}-1-phenylethanol [Ⅰ]
[0022] (R)-Styrene oxide (2.31g, 19.2mmol) and N-benzyl-4-nitrophenethylamine (3.28g, 12.8mmol) in 12ml of isopropanol were refluxed for 20h. The solvent was evaporated under reduced pressure, recrystallized from n-hexane / isopropyl ether, and dried to obtain 3.61 g of yellow solid I, with a yield of 75%. 1 H-NMR (CDCl 3 )δ: 1.28(1H,s) 2.69~2.98(6H,m), 3.57(1H,d,J=13.2Hz), 3.95(1H,d,J=13.2Hz), 4.67~4.69(1H,m) , 7.21~7.36 (12H, m), 8.10~8.12 (2H, m).
Embodiment 2
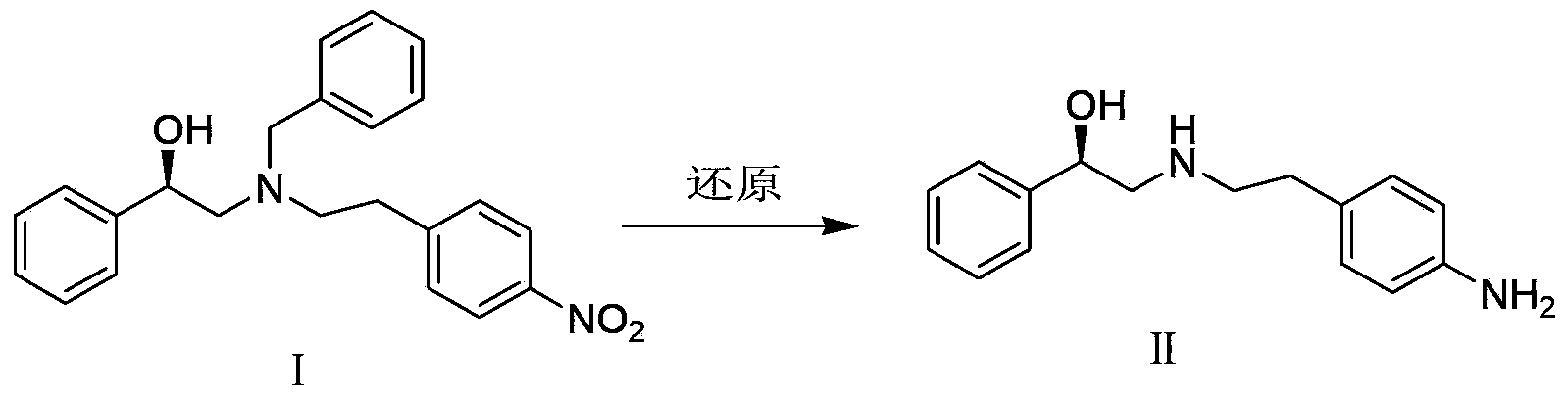
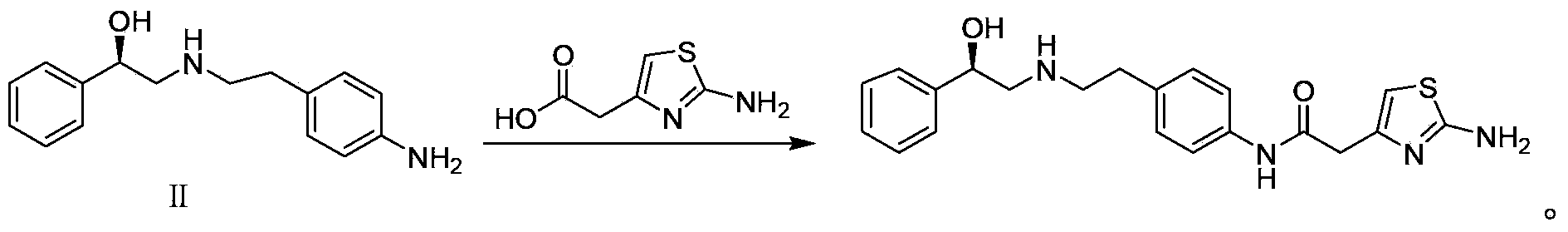
[0023] The preparation of embodiment 2 (R)-2-[(4-aminophenethyl) amino]-1-phenylethanol [Ⅱ]
[0024] Compound Ⅰ (3.0g, 8.0mmol), 10%Pd / C (0.15g) and methanol (25ml) were placed in a hydrogenation reactor, filled with 1MPa hydrogen, reacted at room temperature (10℃~30℃) for 30h, filtered, and the filtrate was concentrated , and dried to give Ⅱ (1.03g, yield 100%, HPLC purity 99.8%).
Embodiment 3
[0025] Preparation of Example 3 (R)-2-[(4-aminophenethyl)amino]-1-phenylethanol [Ⅱ]
[0026] Compound Ⅰ (3.0g, 8.0mmol), 10%Pd / C (0.03g) and methanol (25ml) were placed in a hydrogenation reactor, filled with 10MPa hydrogen, reacted at room temperature (10°C-30°C) for 30h, filtered, and the filtrate was concentrated , and dried to give Ⅱ (1.03g, yield 100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com