Sodium potassium magnesium calcium and glucose injection and preparation method thereof
A technology of glucose injection and injection, which is applied in the direction of medical formulations, aluminum/calcium/magnesium active ingredients, medical preparations of non-active ingredients, etc., can solve the problems of increasing the burden of liver metabolism, respiratory depression, interference, etc., and achieve increased Hepatic metabolic burden, strong buffering effect, and effect of reducing incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
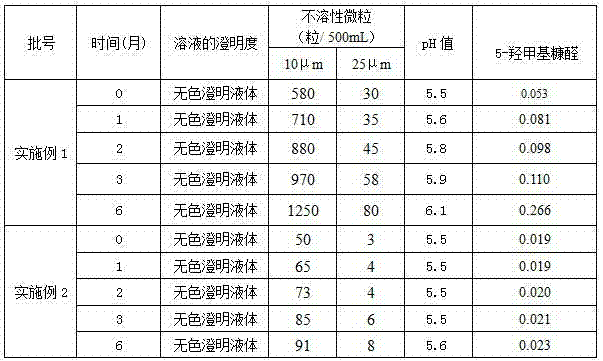
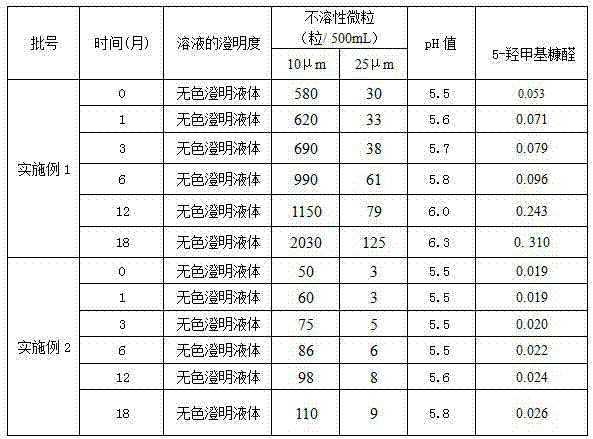
[0019] Sodium Potassium Magnesium Calcium Glucose Injection, every 1000ml injection contains: 6.372g of sodium chloride, 0.30g of potassium chloride, magnesium chloride (MgCl 2 ·6H 2 O) 0.204g, sodium acetate (NaC 2 h 3 o 2 ) 2.052g, sodium citrate (C 6 h 5 Na 3 o 7 2H 2 O) 0.588g, calcium gluconate (C 12 h 22 CaO 14 ·H 2 (0) 0.672g, glucose 10g, and the water for injection of the remainder.
[0020] Preparation:
[0021] (1) Take 70°C water for injection with 80% of the liquid volume, add calcium gluconate and stir to dissolve, then add magnesium chloride, sodium chloride, potassium chloride, sodium acetate, and sodium citrate in sequence, and stir until it is colorless and clear get solution ;
[0022] (2) Add glucose to the solution , stirred until the solution was colorless and clear to obtain a solution ;
[0023] (3) Weigh the solution 0.5% activated carbon by weight, stir well and let it stand for 20 minutes, use a 0.45 μm microporous membrane to ...
Embodiment 2
[0027] Sodium Potassium Magnesium Calcium Glucose Injection, every 1000ml injection contains: 6.372g of sodium chloride, 0.30g of potassium chloride, magnesium chloride (MgCl 2 ·6H 2 O) 0.204g, sodium acetate (NaC 2 h 3 o 2 ) 2.052g, sodium citrate (C 6 h 5 Na 3 o 7 2H 2 O) 0.588g, calcium gluconate (C 12 h 22 CaO 14 ·H 2 (2) 0.672g, glucose 10g, L-leucine 0.25g, and the water for injection of the remainder.
[0028] Preparation:
[0029] (1) Take 70°C water for injection with 80% of the liquid volume, add calcium gluconate and stir to dissolve, then add magnesium chloride, sodium chloride, potassium chloride, sodium acetate, and sodium citrate in sequence, and stir until it is colorless and clear get solution ;
[0030] (2) Add glucose and L-leucine to the solution , stirred until the solution was colorless and clear to obtain a solution ;
[0031] (3) Weigh the solution 0.5% activated carbon by weight, stir well and let it stand for 20 minutes, use a 0...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap