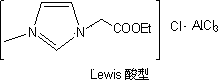
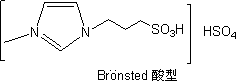Heteropoly ionic liquid catalyst with Br*nsted-Lewis dual acidity
An ionic liquid, dual-acid technology, which is applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc., can solve problems such as low acid strength, and achieve low cost and simple preparation method. , the effect of rich varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] [Example 1] Synthesis of Ionic Liquid 1:
[0038] (1) Weigh 12.21 g (0.10 mol) of 1,3-propane sultone into a three-necked flask (250 mL), add 100 mL of toluene to dissolve it, then add 9.02 g (0.10 mol) of N-methylimidazole, Under magnetic stirring at 50°C, react with nitrogen for 24 h, filter the reaction solution with suction, wash the obtained white precipitate with ethyl acetate three times, and dry it in vacuum at 60°C for 4 h, the obtained white powdery solid is 1-formazol 3-(3-sulfopropyl) imidazolidine sulfonate (MIMPS).
[0039] (2) Weigh Al(NO 3 ) 3 ?9H 2 Add 1.00 g (2.67 mmol) of O and 0.82 g (4 mmol) of MIMPS into a three-neck flask (100 mL), weigh 11.52 g (4 mmol) of phosphotungstic acid, add 40 mL of deionized water to the beaker to dissolve it, and dissolve the phosphorus Add tungstic acid aqueous solution into a three-necked flask, react at room temperature (25 °C) for 24 h under magnetic stirring, distill under reduced pressure, and dry in vacuum at...
Embodiment 2
[0042] [Example 2] Synthesis of ionic liquid 2:
[0043] (1) Weigh 12.21 g (0.10 mol) of 1,3-propane sultone into a three-necked flask (250 mL), add 100 mL of toluene to dissolve it, then add 9.02 g (0.10 mol) of N-methylimidazole, Under magnetic stirring at 50 °C and nitrogen gas for 24 h, the reaction liquid was filtered with suction, the obtained white precipitate was washed three times with ethyl acetate, and vacuum-dried at 60 °C for 4 h, the obtained white powdery solid was MIMPS.
[0044] (2) Weigh Al(NO 3 ) 3 ?9H 2 Add 0.50 g (1.33 mmol) of O and 1.64 g (4 mmol) of MIMPS into a three-necked flask (100 mL), weigh 11.52 g (4 mmol) of phosphotungstic acid, add 40 mL of deionized water to the beaker to dissolve it, and dissolve the phosphorus Add tungstic acid aqueous solution into a three-necked flask, react at room temperature (25 °C) for 24 h under magnetic stirring, distill under reduced pressure, and dry under vacuum at 80 °C for 6 h. The obtained white solid is de...
Embodiment 3
[0047] [Example 3] Synthesis of ionic liquid 3:
[0048] (1) Weigh 12.21 g (0.10 mol) of 1,3-propane sultone into a three-necked flask (250 mL), add 100 mL of toluene to dissolve it, then add 9.02 g (0.10 mol) of N-methylimidazole, Under magnetic stirring at 50 °C and nitrogen gas for 24 h, the reaction liquid was filtered with suction, the obtained white precipitate was washed three times with ethyl acetate, and vacuum-dried at 60 °C for 4 h, the obtained white powdery solid was MIMPS.
[0049] (2) Weigh Al(NO 3 ) 3 ?9H 2 Add 0.50 g (1.33 mmol) of O and 1.64 g (8 mmol) of MIMPS into a three-necked flask (100 mL), weigh 11.52 g (4 mmol) of phosphotungstic acid and add 40 mL of deionized water to the beaker to dissolve it. Add tungstic acid aqueous solution into a three-necked flask, react at room temperature (25 °C) for 24 h under magnetic stirring, distill under reduced pressure, and dry under vacuum at 80 °C for 6 h. The obtained white solid is designated as ionic liquid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com