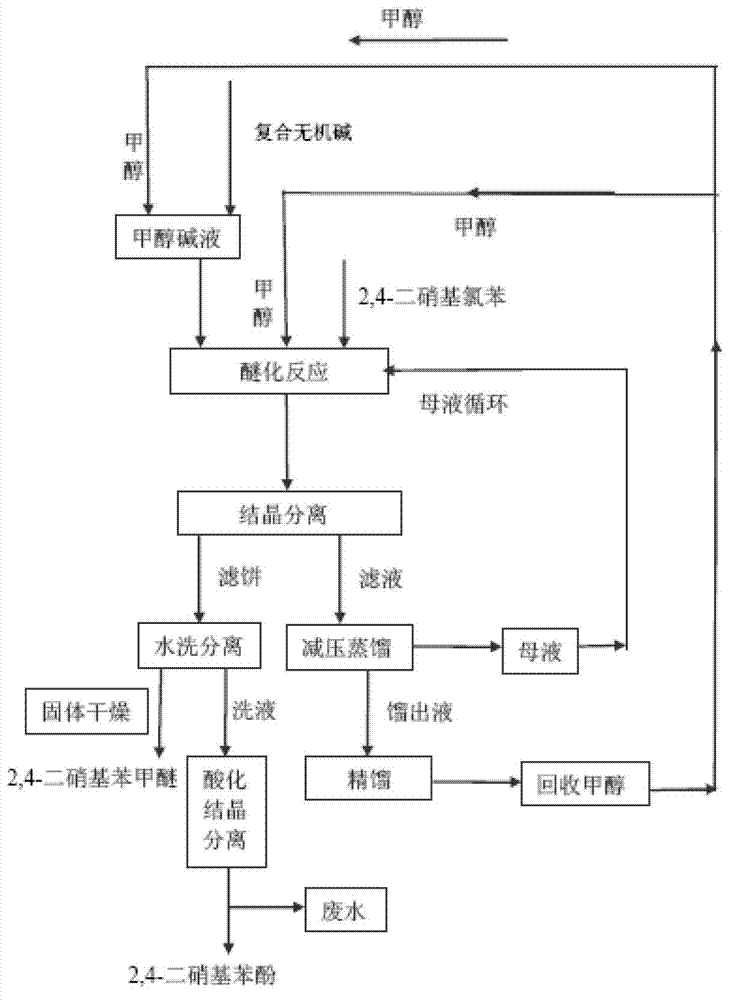
Synthetic process for 2,4-dinitroanisole
A technology of dinitroanisole and synthesis process, which is applied to the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., which can solve problems such as environmental hazards, increased reaction water volume, and reduced yield, and achieve improved reaction Selectivity, reduction of environmental pollution, and reduction of treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 32g (1mol) of methanol into a 150mL four-neck flask, slowly add 16g (0.4mol) of solid sodium hydroxide (0.4mol) and 0.8g (0.02mol) of magnesium oxide under stirring, and control the temperature not to exceed 65°C until the solids are completely dissolved Obtain methanol lye for use.
[0028] (2) Add 45g (1.41mol) of methanol into a 500mL four-neck flask, add 81g (0.4mol) of 2,4-dinitrochlorobenzene under stirring, and heat until 2,4-dinitrochlorobenzene is completely dissolved. Drop the methanol lye obtained in step (1) within 30 minutes, and keep it at 70°C for 3 hours. After cooling to room temperature, suction filtration after the crystals were precipitated, 83 g of the filtrate was collected, and the filtrate was distilled under reduced pressure to obtain 45 g of the mother liquor. The filter cake was washed with water at 30°C until it was neutral, the washing liquid was collected, and the filter cake was dried to obtain 76.4 g of 2,4-dinitroanisole, the yi...
Embodiment 2
[0032] According to the method described in Example 1, 40 g (1.25 mol) of methanol was added in step (1).
[0033] In step (2), 50 g (1.56 mol) of methanol was added, and the reaction was carried out at 72° C. for 5 hours. Obtain 96g of filtrate, 58g of mother liquor after filtrate is distilled under reduced pressure, 76.0g of 2,4-dinitroanisole, take 2,4-dinitrochlorobenzene, the yield is 95.8%, liquid chromatography detects purity 99.8%. 1.77 g of 2,4-dinitrophenol was obtained, calculated as 2,4-dinitrochlorobenzene, the yield was 2.4%, and the purity by liquid chromatography was 99.0%.
[0034]Mother liquor application: According to the method described in Example 1, 40 g (1.25 mol) of methanol was added in step (3).
[0035] In step (4), 58 g of mother liquor and 25.6 g (0.8 mol) of methanol were added, and the mixture was incubated at 72° C. for 5 hours. 108 g of filtrate and 76.5 g of 2,4-dinitroanisole were obtained, calculated as 2,4-dinitrochlorobenzene, the yield...
Embodiment 3
[0037] According to the method described in Example 1, 44.8 g (1.4 mol) of methanol was added in step (1).
[0038] In step (2), 57.6 g (1.8 mol) of methanol was added, and the reaction was carried out at 75° C. for 6 hours. Obtain filtrate 110g, the mother liquor 70g after filtrate is distilled under reduced pressure, obtain 2,4-dinitroanisole 75.3g, calculate with 2,4-dinitrochlorobenzene, yield is 95%, liquid chromatography detects 99.5% purity. 2.12 g of 2,4-dinitrophenol was obtained, calculated as 2,4-dinitrochlorobenzene, the yield was 2.88%, and the purity by liquid chromatography was 98.3%.
[0039] Mother liquor application: According to the method described in Example 1, 44.8 g (1.4 mol) of methanol was added in step (3).
[0040] In step (4), 70 g of mother liquor and 28.8 g (1.8 mol) of methanol were added, and the mixture was incubated at 75°C for 6 hours. Obtain filtrate 105g, the mother liquor 70g after filtrate is distilled under reduced pressure, obtain 2,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com